An experimental study on detonation characteristics of binary fuels hydrogen/propane-air mixtures
-
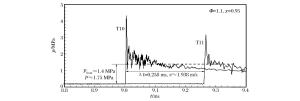
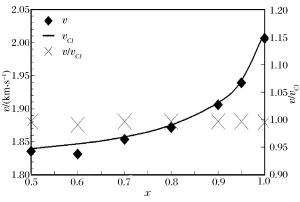
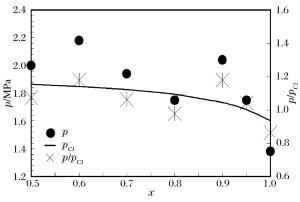
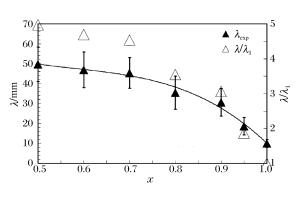
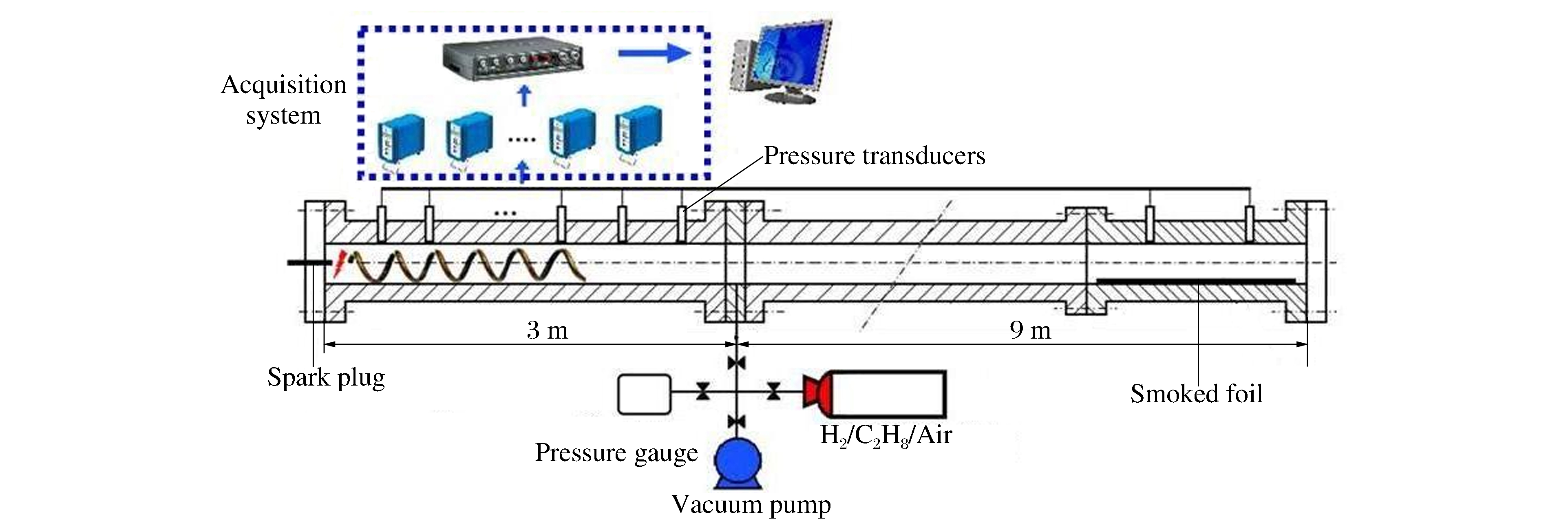
摘要: 通过采用压力传感器和烟灰板两种测试设备,开展了常温常压下氢气/丙烷和空气混合气体爆轰性能的实验研究。实验过程中观察到自持爆轰波,爆轰速度比值在0.99~1之间,爆轰压力比值在0.8~1.2之间。爆轰胞格尺寸在10~50 mm范围内,建立了爆轰胞格尺寸和化学诱导长度的关系式。随着丙烷不断添加,爆轰速度减小,而爆轰压力和胞格尺寸增加。这种变化趋势起初较快,而后变缓。因为起初氢气摩尔分数较大,混合气体趋向于氢气/空气的爆轰性能;而后因丙烷摩尔质量较大,丙烷逐渐起主要作用,混合气体表现出丙烷/空气的爆轰性能。Abstract: The paper is aimed to experimentally probe the detonation characteristics of the binary fuel hydrogen/propane-air mixture. The experiments were conducted in an obstructed cylindrical tube with a 92-mm inner diameter and a 12-m length at normal pressure and temperature. Eleven instrument ports and eleven piezoelectric pressure transducers were adopted on the tube wall surface. A Schelkin spiral with a blockage ratio of 0.5 and a pitch with inner diameter as the tube and with the length of 3 m were used to accelerate the flame propagation until the detonation initiated. The studied binary fuel mixtures with equivalence ratio of 1.1 and hydrogen molar fraction varying from 0.5 to 1.0 were prepared by the partial pressure and ignited via a spark plug at about 15-mJ discharge energy. The detonation characteristic parameters such as velocity, pressure and cell size were achieved with pressure transducers and smoking foils, respectively. It can be therefore concluded that the self-sustained detonation is observed as follows: (ⅰ) detonation velocity ratiov/vCJ varies from 0.99 to 1.0 and pressure ratio p/pCJ changes from 0.8 to 1.2; (ⅱ) detonation cell size varies from 10 mm to 50 mm. When propane is added to hydrogen/air mixtures, the detonation velocity decreases, but the pressure and cell size inversely increase. The variation trends of the detonation parameters at the beginning change quickly because the detonation characteristics of hydrogen/propane-air mixtures are similar to those of hydrogen/air due to the larger hydrogen molar fraction. Afterwards, the trends gradually slow down because the increasing molar fraction of propane with heavier molecular mass in the mixtures which plays a dominant role in the binary fuels. At last, a relationship between detonation cell size and ZND chemical induction length was obtained. Thus, our conclusion can provide the experimental data in the hydrogen explosion hazard prevention.
-
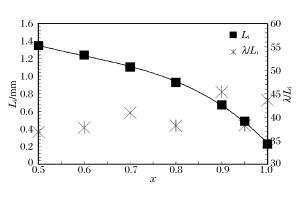
表 1 混合气体爆轰性能参数的理论值
Table 1. CJ detonation theoretical values of the studied mixtures
x w(H2)/% w(C3H8)/% vCJ/(km·s-1) pCJ/MPa Li/mm 0.5 4.35 95.65 1.839 1.875 1.348 3 0.6 6.38 93.62 1.846 1.849 1.243 0 0.7 9.59 90.41 1.857 1.827 1.106 6 0.8 15.38 84.62 1.874 1.792 0.928 3 0.9 29.03 70.97 1.909 1.732 0.674 7 0.95 46.34 53.66 1.942 1.681 0.487 9 1.0 100.00 0 2.015 1.599 0.229 1 -
[1] Desbordes D. A study of deflagration-to-detonation transition[R]. Poitiers, France: Laboratory of Combustion and Detonation, 1993. [2] Ciccarelli G, Dorofeev S B. Flame acceleration and transition to detonation in ducts[J]. Progress in Energy and Combustion Science, 2008, 34(4): 499-550. https://www.sciencedirect.com/science/article/pii/S0360128507000639 [3] 卢捷, 宁建国, 王成, 等.煤气火焰传播规律及其加速机理研究[J].爆炸与冲击, 2004, 24(4): 305-311. http://www.bzycj.cn/article/id/9960Lu Jie, Ning Jian-guo, Wang Cheng, et al. Study on flame propagation and acceleration mechanism of city coal gas[J]. Explosion and Shock Waves, 2004, 24(4): 305-311. http://www.bzycj.cn/article/id/9960 [4] Law C K, Kwon O C. Effects of hydrocarbon substitution on atmospheric hydrogen-air flame propagation[J]. International Journal of Hydrogen and Energy, 2004, 29(8): 867-879. https://www.sciencedirect.com/science/article/pii/S0360319903002519 [5] Tang C L, Huang Z H, Jin C, et al. Laminar burning velocities and combustion characteristics of propane-hydrogen-air premixed flame[J]. International Journal of Hydrogen and Energy, 2008, 33(18): 4906-4914. https://www.sciencedirect.com/science/article/pii/S0360319908007702 [6] Takita K, Niioka T. On detonation behavior of mixed fuels[J]. Shock Waves, 1996, 6(2): 16-66. doi: 10.1007/BF02515188 [7] Matignon C. Etude de la détonation de deux mélanges stoechiométriques(H2 /CH4/O2 /N2 et CH4/C2H6/O2 /N2): Influence de la proportion relative des deux combustibles et de la températur initiale élevée[D]. Poitiers: University of Poitiers, 2000. [8] Bozier O, Sorin R, Zitoun R, et al. Detonation characteristics of H2-natural gas-air mixtures[C]//Proceeding of European Combustion Meeting. Vienna, Austria: German Section of the Combustion Institute, 2009: 14-17. [9] Sorin R, Bozier O, Zitoun R, et al. Deflagration to detonation transition in binary fuels H2/CH4 with air mixtures[C]//Proceeding of 22nd ICDERS. Minsk, Belarus: Heat and Mass Transfer Institute of National Academy of Science of Belarus, 2009: 27-31. [10] Chaumeix N, Pichon S, Lafosse F, et al. Role of chemical kinetics on the detonation properties of hydrogen /natural gas/air mixtures[J]. International Journal of Hydrogen and Energy, 2007, 32(13): 2216-2226. https://www.sciencedirect.com/science/article/pii/S0360319907002145 [11] 孙锦山, 朱建士.理论爆轰物理[M].北京: 国防工业出版社, 1995. [12] Smith G P, Golden D, Frenklach M, et al. GRI-Mech 3.0[Z]. 1999. -






 下载:
下载: