Effects of temperature and concentration on characteristic parameters of methanol explosion
-
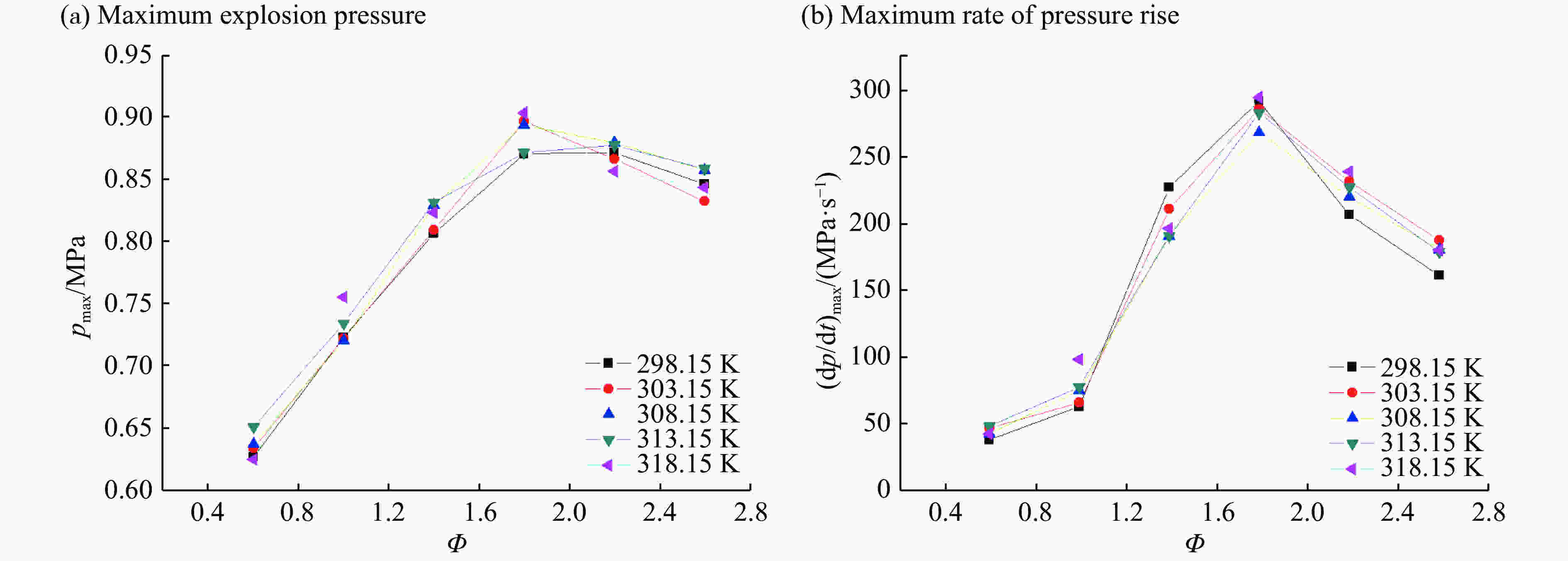
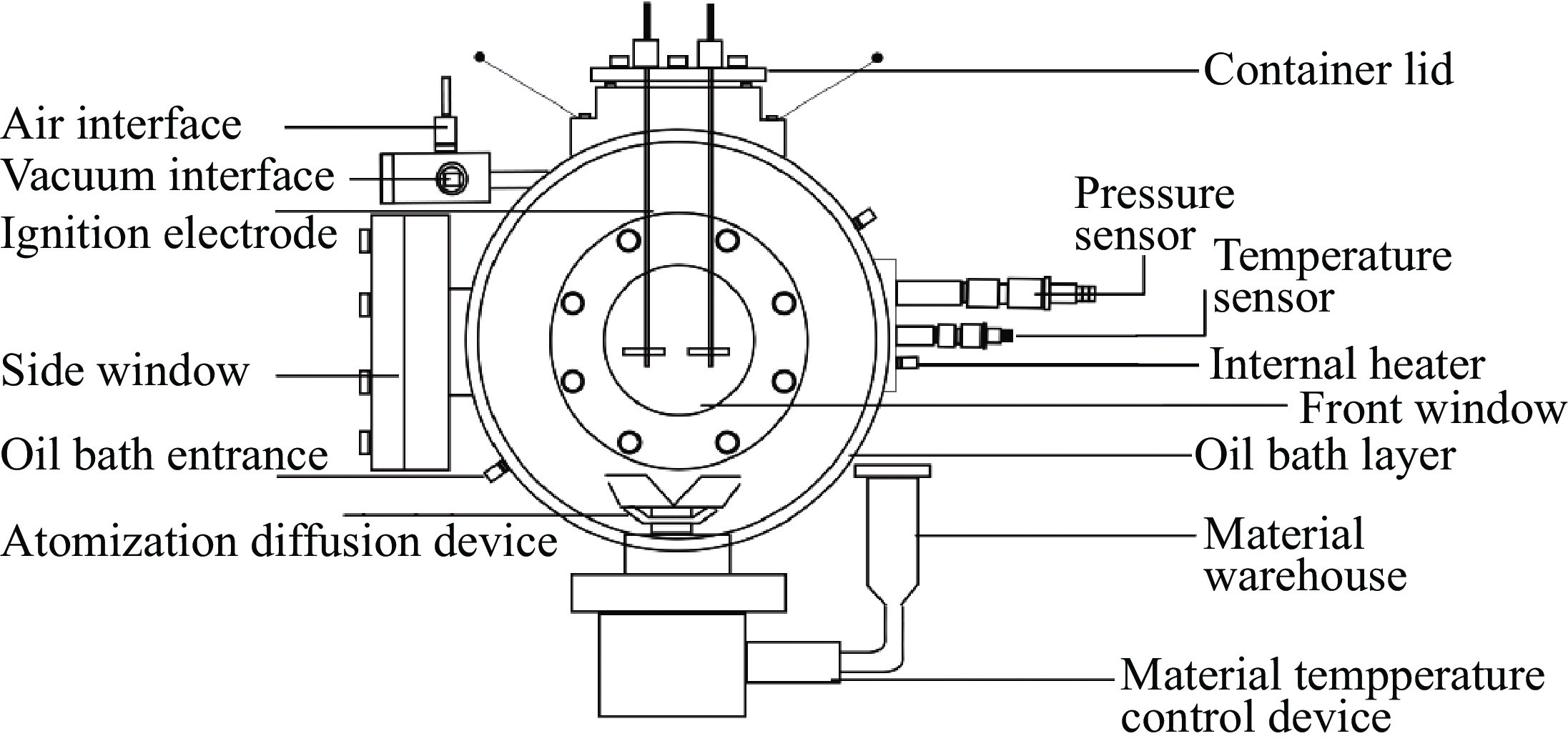
摘要: 采用20 L球形喷雾爆炸实验系统,探究甲醇在不同环境温度、物料温度及喷雾浓度下的爆炸特性规律。结果表明:20 L爆炸球内甲醇喷雾液滴爆炸极限范围为118.8~594.0 g/cm3,与纯气相爆炸极限范围(78.6~628.6 g/cm3)相比,甲醇喷雾液滴爆炸极限范围较窄,喷雾液滴的爆炸敏感性比纯气相甲醇蒸汽低。随着爆炸球内环境温度的升高,甲醇喷雾爆炸极限范围变宽,受限空间内甲醇气液喷雾点火成功概率增大。当甲醇物料自身温度或爆炸容器内环境温度保持不变时,相应爆炸特性参数在Φ=1.8拐点处均呈现先增大后减小的趋势。当Φ=1.8时,甲醇喷雾爆炸存在最大超压峰值。环境温度、物料温度的升高可以提高雾化质量,促进扩散燃烧。但是环境温度的变化较之物料温度的改变对于甲醇液滴喷雾爆炸特性参数的影响更为显著。环境温度和化学当量比二元变量共同影响着甲醇喷雾爆炸强度值,当Φ=1.8,环境温度为303.15 K时,甲醇的喷雾爆炸强度大于甲烷气体爆炸的爆炸强度。Abstract: The explosion characteristics of methanol under different ambient temperature, material temperature and spray concentration were investigated by using the 20 L spherical explosion experiment system. The results show that the explosion limit of methanol spray droplets in the 20 L explosion vessel is 118.8−594.0 g/cm3. Compared with the limit range of pure gas explosion (78.6−628.6 g/cm3), the limit range of methanol spray droplets explosion is narrower, and the sensitivity of spray droplets is lower than that of pure gas methanol vapor. As the ambient temperature in the explosion vessel increases, the limit range of methanol spray explosion becomes wider, and the probability of successful ignition of gas-liquid spray in the confined space increases. When the temperature of the methanol or the ambient temperature of the explosion vessel remains unchanged, parameters of the corresponding explosion characteristic firstly increase, and then decrease after the inflection point of Φ=1.8. When Φ=1.8, there is a maximum over pressure peak in the methanol spray explosion. The increasing ambient temperature and material temperature can improve the atomization and then promote diffusion combustion. However, the effect of ambient temperature is more significant than the factor of material temperature on the characteristic parameters of methanol droplet spray explosion. The ambient temperature and stoichiometric ratio affect the strength value of methanol spray explosion. When Φ=1.8 and the ambient temperature is 303.15 K, the intensity of methanol spray explosion is greater than the intensity of methane gas explosion.
-
Key words:
- methanol /
- material temperature /
- ambient temperature /
- explosion index /
- explosion limit
-
表 1 甲醇气-液两相浓度随爆炸球内环境温度变化关系
Table 1. Relation between the methanol concentrations in vapor/liquid phaseand the ambient temperature in 20 L spherical vessel
甲醇喷雾化学当量比 环境温度/K 总浓度/(g·cm−3) 气相质量浓度/(g·cm−3) 液相质量浓度/(g·cm−3) 0.2 298.15 39.6 0.217 39.382 0.2 303.15 39.6 0.276 39.323 0.2 308.15 39.6 0.348 39.251 0.2 313.15 39.6 0.435 39.165 0.2 318.15 39.6 0.538 39.061 0.6 298.15 118.8 0.217 118.583 0.6 303.15 118.8 0.276 118.524 0.6 308.15 118.8 0.348 118.452 0.6 313.15 118.8 0.435 118.365 0.6 318.15 118.8 0.538 118.262 1.0 298.15 198.0 0.217 197.783 1.0 303.15 198.0 0.276 197.724 1.0 308.15 198.0 0.348 197.652 1.0 313.15 198.0 0.435 197.565 1.0 318.15 198.0 0.538 197.462 1.4 298.15 277.2 0.217 276.983 1.4 303.15 277.2 0.276 276.924 1.4 308.15 277.2 0.348 276.852 1.4 313.15 277.2 0.435 276.765 1.4 318.15 277.2 0.538 276.662 1.8 298.15 356.4 0.217 356.183 1.8 303.15 356.4 0.276 356.124 1.8 308.15 356.4 0.348 356.052 1.8 313.15 356.4 0.435 355.965 1.8 318.15 356.4 0.538 355.862 2.2 298.15 435.6 0.217 435.383 2.2 303.15 435.6 0.276 435.324 2.2 308.15 435.6 0.348 435.252 续表 1 甲醇喷雾化学当量比 环境温度/K 总浓度/(g·cm−3) 气相质量浓度/(g·cm−3) 液相质量浓度/(g·cm−3) 2.2 313.15 435.6 0.435 435.165 2.2 318.15 435.6 0.538 435.062 2.6 298.15 514.8 0.217 514.583 2.6 303.15 514.8 0.276 514.524 2.6 308.15 514.8 0.348 514.452 2.6 313.15 514.8 0.435 514.365 2.6 318.15 514.8 0.538 514.262 3.0 298.15 594.0 0.217 593.783 3.0 303.15 594.0 0.276 593.724 3.0 308.15 594.0 0.348 593.652 3.0 313.15 594.0 0.435 593.565 3.0 318.15 594.0 0.538 593.462 表 2 爆炸容器内环境温度对甲醇爆炸极限的影响
Table 2. Effect of ambient temperature on explosion limit of methanol explosion
甲醇喷雾化学当量比 质量浓度/(g·cm−3) 环境温度/K 是否点火成功 超压峰值/MPa 超压上升速率峰值/(MPa·s−1) 0.2 39.6 298.15 否 − − 0.2 39.6 303.15 否 − − 0.2 39.6 308.15 否 − − 0.2 39.6 313.15 否 − − 0.2 39.6 318.15 否 − − 0.6 118.8 298.15 否 − − 0.6 118.8 303.15 否 − − 0.6 118.8 308.15 是 0.627 38.146 0.6 118.8 313.15 是 0.637 39.613 0.6 118.8 318.15 是 0.662 63.087 3.0 594.0 298.15 否 − − 3.0 594.0 303.15 否 − − 3.0 594.0 308.15 否 − − 3.0 594.0 313.15 是 0.764 105.643 3.0 594.0 318.15 是 0.794 146.714 表 3 环境温度对爆炸指数的影响
Table 3. Effects of ambient temperature on explosion index of methanol explosion
环境温度/K 甲醇喷雾化学当量比 爆炸指数/(MPa·m·s−1) 环境温度/K 甲醇喷雾化学当量比 爆炸指数/(MPa·m·s−1) 308.15 0.6 11.549 303.15 1.8 64.913 313.15 0.6 12.744 308.15 1.8 72.878 318.15 0.6 14.337 313.15 1.8 80.047 298.15 1.0 11.547 318.15 1.8 99.560 303.15 1.0 14.337 298.15 2.2 39.824 308.15 1.0 20.310 303.15 2.2 49.983 313.15 1.0 37.036 308.15 2.2 59.736 318.15 1.0 48.586 313.15 2.2 70.887 298.15 1.4 28.275 318.15 2.2 82.834 303.15 1.4 39.426 298.15 2.6 37.035 308.15 1.4 51.772 303.15 2.6 46.992 313.15 1.4 64.515 308.15 2.6 48.984 318.15 1.4 70.489 313.15 2.6 68.099 298.15 1.8 44.603 318.15 2.6 80.047 -
[1] BEECKMANN J, CAI L, PITSCH H. Experimental investigation of the laminar burning velocities of methanol, ethanol, n-propanol, and n-butanol at high pressure [J]. Fuel, 2014, 117: 340–350. DOI: 10.1016/j.fuel.2013.09.025. [2] ZHANG X, WANG G, ZOU J, et al. Investigation on the oxidation chemistry of methanol in laminar premixed flames [J]. Combustion and Flame, 2017, 180: 20–31. DOI: 10.1016/j.combustflame.2017.02.016. [3] SAEED K. Determination of the explosion characteristics of methanol-Air mixture in a constant volume vessel [J]. Fuel, 2017, 210: 729–737. DOI: 10.1016/j.fuel.2017.09.004. [4] MITU M, BRANDES E. Explosion parameters of methanol-air mixtures [J]. Fuel, 2015, 158: 217–223. DOI: 10.1016/j.fuel.2015.05.024. [5] GRABARCZYK M, TEODORCZYK A, DI SARLI V, et al. Effect of initial temperature on the explosion pressure of various liquid fuels and their blends [J]. Journal of Loss Prevention in the Process Industries, 2016, 44: 775–779. DOI: 10.1016/j.jlp.2016.08.013. [6] 孙彦龙, 谭迎新, 谢溢月, 等. 甲醇汽油混合物爆炸下限测试研究 [J]. 中国安全科学学报, 2015, 25(12): 56–61. DOI: 10.16265/j.cnki.issn1003-3033.2015.12.010.SUN Yanlong, TAN Yingxin, XIE Yiyue, et al. Study on lower explosive limits of methanol-gasoline blends [J]. China Safety Science Journal, 2015, 25(12): 56–61. DOI: 10.16265/j.cnki.issn1003-3033.2015.12.010. [7] 刘金彪, 谭迎新, 于金升, 等. 氮气与二氧化碳对甲醇爆炸极限的影响 [J]. 测试技术学报, 2017, 31(6): 546–550.LIU Jinbiao, TAN Yingxin, YU Jinsheng, et al. Influence of nitrogen and carbon dioxide on methanol explosion limit [J]. Journal of Test and Measurement Technology, 2017, 31(6): 546–550. [8] 陈长坤, 王玮玉, 刘晅亚. 隧道内甲醇液体蒸发及蒸气扩散规律数值模拟分析 [J]. 中国安全生产科学技术, 2017, 13(12): 52–57.CHEN Changkun, WANG Weiyu, LIU Xuanya. Numerical simulation analysis on evaporation of methanol liquid and diffusion laws of methanol vapor in tunnel [J]. Journal of Safety Science and Technology, 2017, 13(12): 52–57. [9] 姚春德, 陈志方, 吴涛阳, 等. 甲醇温度和压力对喷雾特性的影响试验 [J]. 农业机械学报, 2015, 46(11): 377–382. DOI: 10.6041/j.issn.1000-1298.2015.11.051.YAO Chunde, CHEN Zhifang, WU Taoyang, et al. Experiment on effects of methanol temperature and pressure on spray [J]. Transactions of the Chinese Society for Agricultural Machinery, 2015, 46(11): 377–382. DOI: 10.6041/j.issn.1000-1298.2015.11.051. [10] 姚春德, 陈志方, 银增辉, 等. 燃油温度和环境温度对甲醇低压喷雾的影响 [J]. 内燃机学报, 2015, 33(4): 310–315. DOI: 10.16236/j.cnki.nrjxb.201504044.YAO Chunde, CHEN Zhifang, YIN Zenghui, et al. Effect of fuel and environmental temperature on the low pressure methanol spray [J]. Transactions of CSICE, 2015, 33(4): 310–315. DOI: 10.16236/j.cnki.nrjxb.201504044. [11] 王悦, 白春华. 乙醚云雾场燃爆参数实验研究 [J]. 爆炸与冲击, 2016, 36(4): 497–502. DOI: 10.11883/1001-1455(2016)04-0497-06.WANG Yue, BAI Chunhua. Experimental research on explosion parameters of diethyl ether mist [J]. Explosion and Shock Waves, 2016, 36(4): 497–502. DOI: 10.11883/1001-1455(2016)04-0497-06. [12] 王悦. 可燃液体燃料云雾形成和爆炸问题研究 [D]. 北京: 北京理工大学, 2016: 44−45. [13] 张英华, 黄志安, 高玉坤. 燃烧与爆炸学 [M]. 第2版. 北京: 冶金工业出版社, 2015: 223−224. [14] 蒋军成. 化工安全 [M]. 北京: 机械工业出版社, 2008: 60−65. -







 下载:
下载: