Effect of inert gas addition on syngas explosion
-
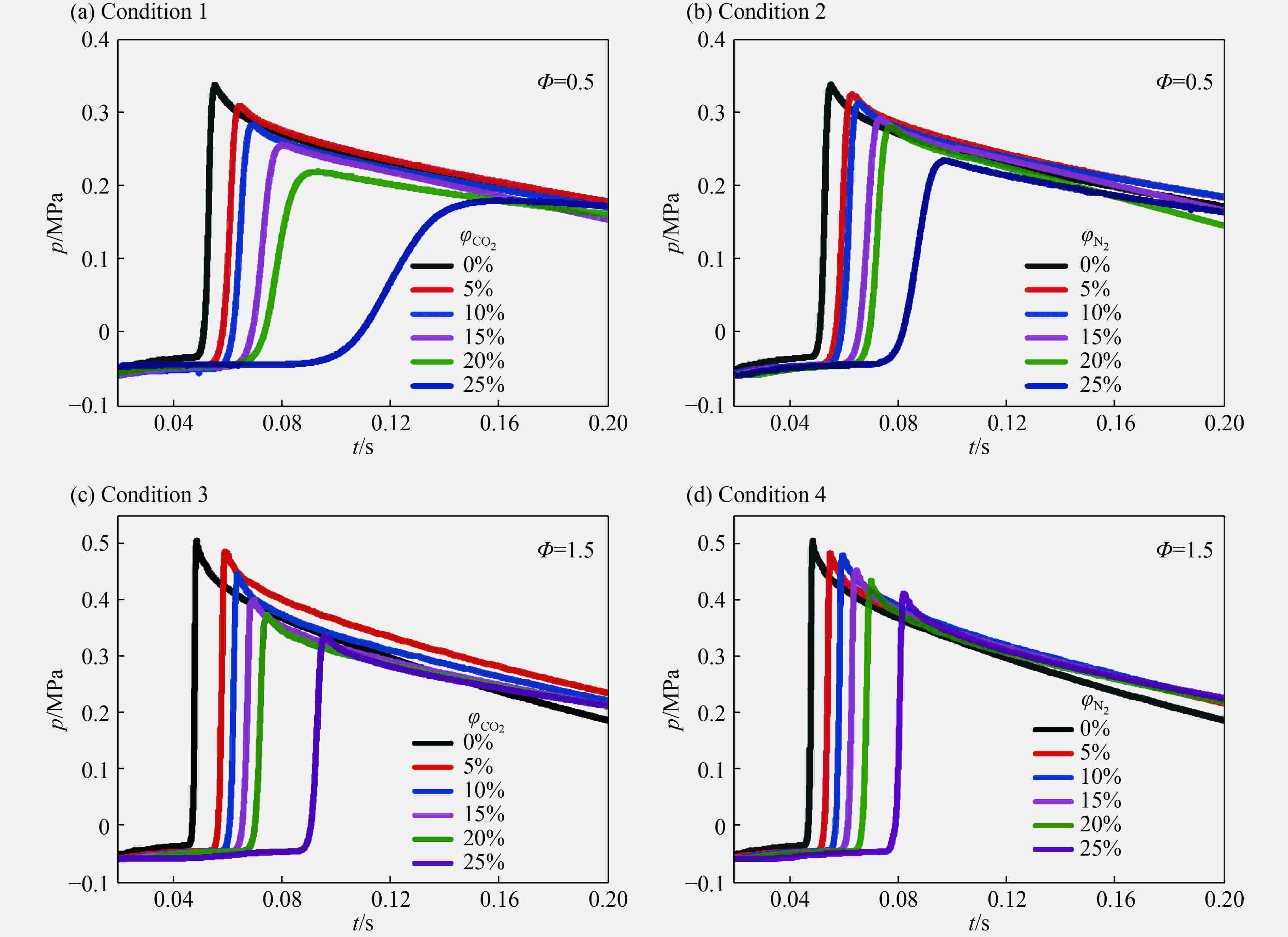
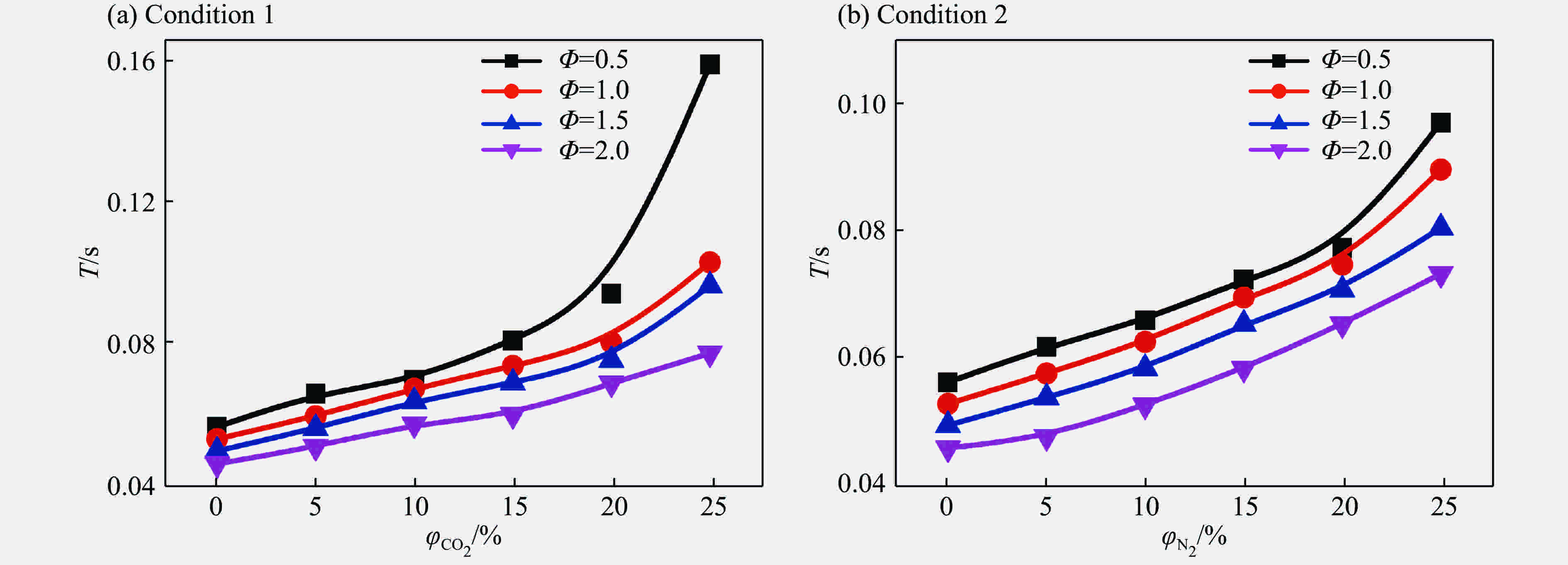
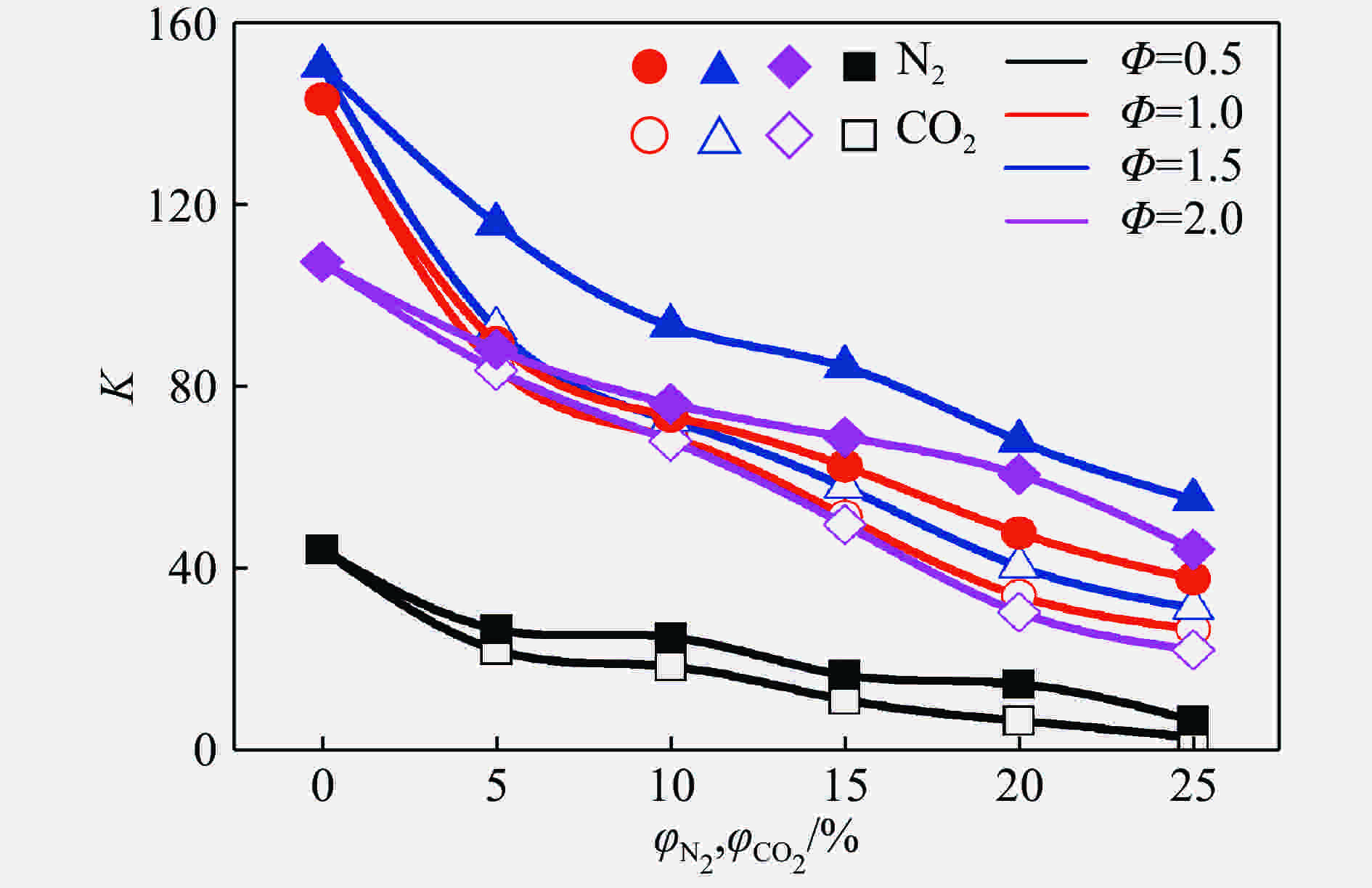
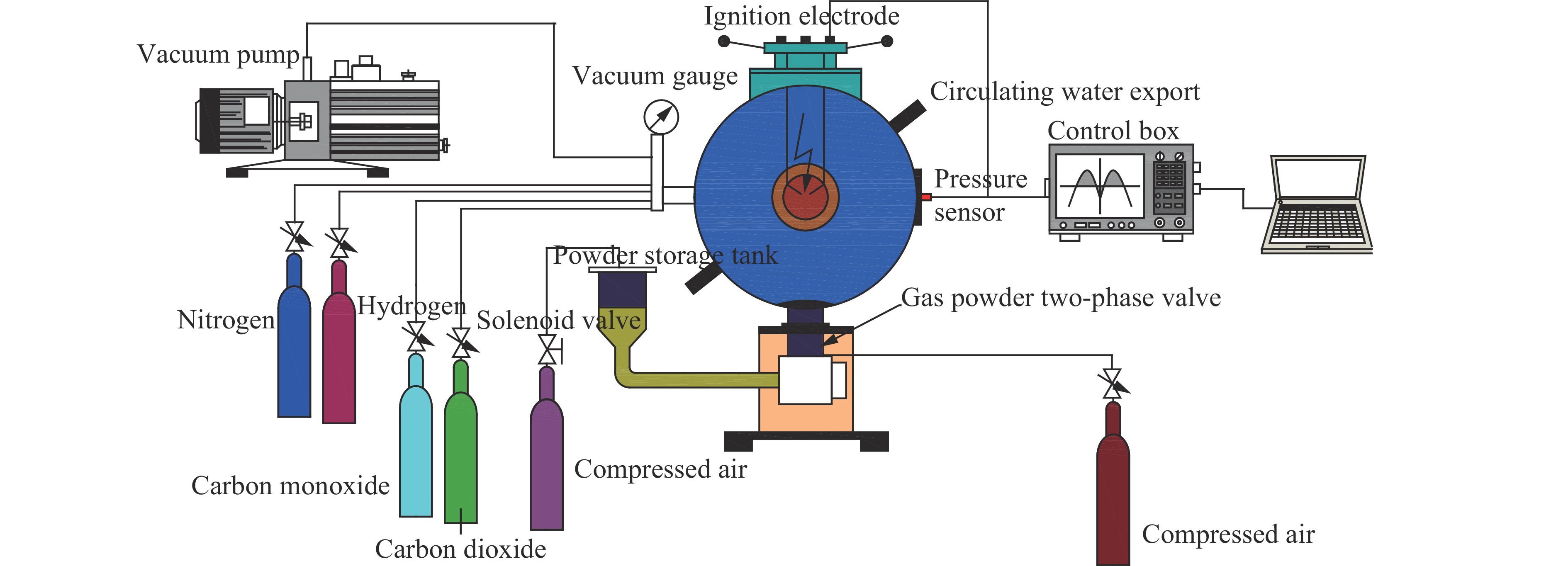
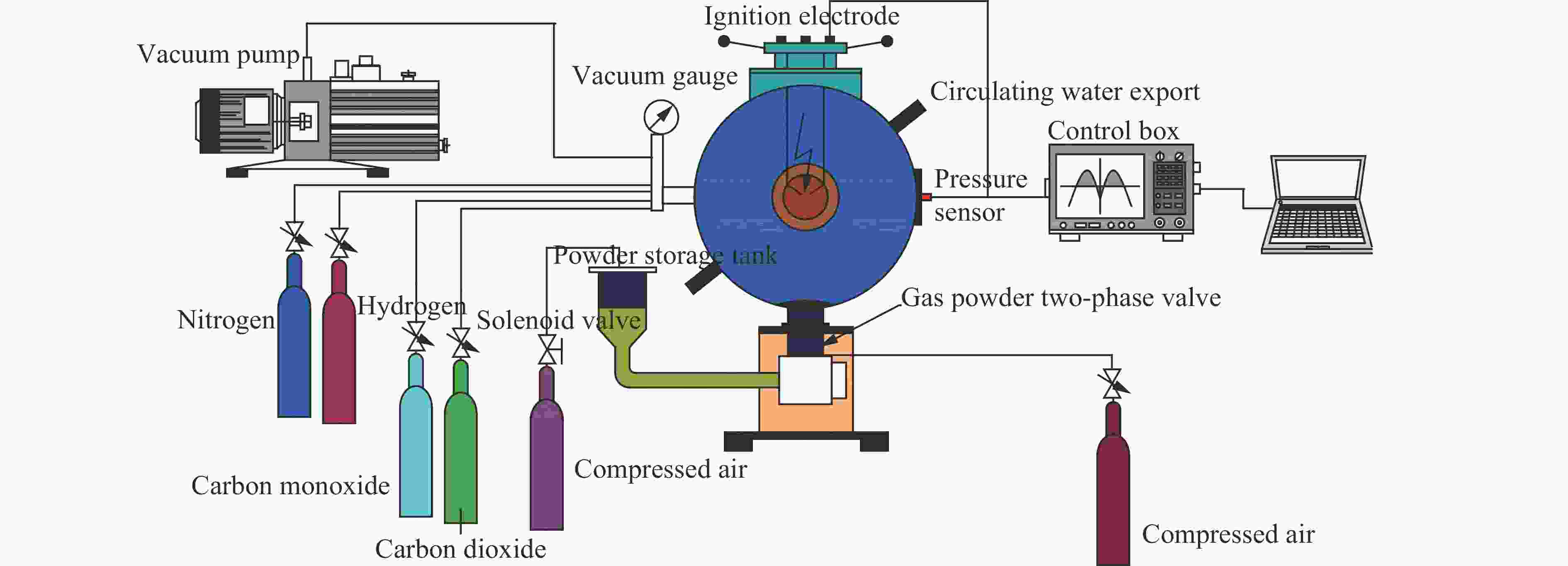
摘要: 为了研究惰性气体(氮气及二氧化碳)对合成气爆炸特性的影响,利用20 L球形爆炸仪器,开展不同体积分数氮气与二氧化碳作用下不同当量比合成气的爆炸实验,从爆炸峰值压力、爆炸压力到达峰值时间、爆炸指数方面分析惰性气体对合成气爆炸特性的影响。研究结果表明:惰性气体体积分数的增加会降低合成气的爆炸压力和爆炸指数,推迟爆炸压力到达峰值的时间;在相同体积分数下,CO2比N2能更有效地降低合成气的爆炸峰值压力和爆炸指数,减小爆炸反应的剧烈程度,CO2在抑制合成气爆炸方面比N2的效果明显。Abstract: In this study we investigated the effect of inert gas addition on the characteristics of the syngas explosion using 20 L spherical explosive device. Effects of different volume fraction of inert gas (CO2/N2) on the explosion parameters including the peak pressure, the delay of the peak pressure time, and the explosion index were obtained from the experiment. The results show that the delay of the peak pressure time of the syngas explosion rose higher, and the explosion peak pressure and the explosion index fell lower with the increase of the volume fraction of the inert gas; that CO2 had a stronger inhibition effect on syngas explosion than N2 because the peak pressure and the explosion index fell down more sharply with the addition of CO2 than of N2.
-
Key words:
- syngas /
- explosion pressure /
- equivalence ratio /
- inert gas
-
表 1 不同实验工况下气体体积分数
Table 1. Volume fraction of gases under different experimental conditions
$ {{\textit{φ}}_{\rm{inert}}} $/% $ {\textit{φ}}_{{{\rm H}_{2}}}\!,{{\textit{φ}}_{\rm{CO}}} $/% ${\textit{φ}}_{\rm{air}} $/% ${\textit{φ}}_{{{\rm H}_{2}}}\!,{{\textit{φ}}_{\rm{CO}}} $/% ${\textit{φ}}_{\rm{air}} $/% ${\textit{φ}}_{{{\rm H}_2}}\!,{{\textit{φ}}_{\rm{CO}}} $/% ${\textit{φ}}_{\rm{air}} $/% ${\textit{φ}}_{{{\rm H}_2}} \!,{{\textit{φ}}_{\rm{CO}}}$/% ${\textit{φ}}_{\rm{air}} $/% Φ=0.5 Φ=1.0 Φ=1.5 Φ=2.0 0 8.68 82.64 14.79 70.42 19.33 61.34 22.83 54.34 5.00 8.25 78.50 14.05 66.90 18.36 58.28 21.69 51.62 10.00 7.81 74.38 13.31 63.38 17.39 55.22 20.55 48.90 15.00 7.38 70.24 12.57 59.86 16.43 52.14 19.41 46.19 20.00 6.94 66.12 11.83 56.34 15.46 49.08 18.26 43.48 25.00 6.51 61.98 11.09 52.82 14.49 46.02 17.12 40.76 表 2 添加惰性气体后峰值压力下降值
Table 2. Decrease of peak pressure during syngas explosion with inert gas
Φ 惰性气体 Δpmax/MPa 5% 10% 15% 20% 25% 0.5 N2 0.009 37 0.021 56 0.042 67 0.053 65 0.100 34 CO2 0.025 12 0.051 37 0.077 72 0.114 30 0.153 89 1.0 N2 0.002 57 0.011 98 0.028 53 0.047 04 0.062 88 CO2 0.019 07 0.044 82 0.073 77 0.104 40 0.123 47 1.5 N2 0.012 55 0.020 56 0.043 34 0.073 25 0.084 23 CO2 0.020 11 0.056 38 0.096 89 0.123 86 0.161 27 2.0 N2 0.020 05 0.037 43 0.057 97 0.076 6 0.091 45 CO2 0.043 07 0.070 91 0.112 81 0.137 91 0.143 39 表 3 添加惰性气体后压力到达峰值时间的延迟
Table 3. Delay of peak pressure time during syngas explosion with inert gas
Φ 惰性气体 ΔT/s 5% 10% 15% 20% 25% 0.5 N2 0.002 4 0.007 2 0.010 0 0.015 2 0.027 7 CO2 0.004 2 0.009 4 0.011 4 0.024 8 0.037 6 1.0 N2 0.004 8 0.007 8 0.012 8 0.018 0 0.022 0 CO2 0.009 4 0.012 2 0.015 6 0.022 2 0.026 8 1.5 N2 0.002 6 0.004 0 0.007 4 0.014 0 0.017 0 CO2 0.006 4 0.009 4 0.013 2 0.016 4 0.019 5 2.0 N2 0.001 8 0.004 8 0.008 6 0.014 6 0.017 5 CO2 0.007 8 0.011 2 0.013 8 0.015 7 0.021 2 表 4 添加N2后爆炸指数相比于添加CO2后爆炸指数的差值
Table 4. Difference between explosion indexeswith N2 and CO2
Φ ΔK 5% 10% 15% 20% 25% 0.5 4.546 12 5.364 57 6.455 34 8.001 46 3.636 89 1.0 5.092 24 7.546 12 10.912 15 13.641 28 10.909 22 1.5 22.727 66 24.549 04 26.185 94 27.276 72 23.641 28 2.0 4.546 12 8.183 02 19.095 16 30.004 39 21.822 83 -
[1] JO Y D, CROWL D A. Explosion characteristics of hydrogen-air mixtures in a spherical vessel [J]. Process Safety Progress, 2010, 29(3): 216–23. DOI: 10.1002/prs.10370. [2] TANG C, HUANG Z, JIN C, et al. Explosion characteristics of hydrogen-nitrogen-air mixtures at elevated pressures and temperatures [J]. International Journal of Hydrogen Energy, 2009, 34(1): 554–561. DOI: 10.1016/j.ijhydene.2008.10.028. [3] CAMMAROTA F, SARLI V D, SALZANO E, et al. Measurements of pressure and flame speed during explosions of CH4/O2/N2/CO2 mixtures [J]. Journal of Loss Prevention in the Process Industries, 2016, 44: 771–774. DOI: 10.1016/j.jlp.2016.06.005. [4] BURBANO H J, PAREJA J, AMELL A A. Laminar burning velocities and flame stability analysis of H2/CO/air mixtures with dilution of N2 and CO2 [J]. International Journal of Hydrogen Energy, 2011, 36(4): 3232–3242. DOI: 10.1016/j.ijhydene.2010.11.089. [5] VU T M, PARK J, KWON O B, et al. Effects of diluents on cellular instabilities in outwardly propagating spherical syngas-air premixed flames [J]. International Journal of Hydrogen Energy, 2010, 35(8): 3868–3880. DOI: 10.1016/j.ijhydene.2010.01.091. [6] PRATHAP C, RAY A, RAVI M R. Effects of dilution with carbon dioxide on the laminar burning velocity and flame stability of H2-CO mixtures at atmospheric condition [J]. Combustion and Flame, 2012, 159(2): 482–492. DOI: 10.1016/j.combustflame.2011.08.006. [7] XIE Y, WANG J, XU N, et al. Comparative study on the effect of CO2 and H2O dilution on laminar burning characteristics of CO/H2/air mixtures [J]. International Journal of Hydrogen Energy, 2014, 39(7): 3450–3458. DOI: 10.1016/j.ijhydene.2013.12.037. [8] 孙绍增, 孟顺, 赵义军, 等. 水蒸气纯氧条件下合成气燃烧特性 [J]. 化工学报, 2015, 66(12): 5119–5126. DOI: 10.11949/j.issn.0438-1157.20150725.SUN Shaozeng, MENG Shun, ZHAO Yijun, et al. Combustion characteristics of syngas under oxygen steam conditions [J]. CIESC Journal, 2015, 66(12): 5119–5126. DOI: 10.11949/j.issn.0438-1157.20150725. [9] WANG Z H, WENG W B, HE Y, et al. Effect of H2/CO ratio and N2/CO2 dilution rate on laminar burning velocity of syngas investigated by direct measurement and simulation [J]. Fuel, 2015, 141(1): 285–292. DOI: 10.1016/j.fuel.2014.10.040. [10] ZHANG Y, SHEN W, ZHANG H, et al. Effects of inert dilution on the propagation and extinction of lean premixed syngas/air flames [J]. Fuel, 2015, 157: 115–121. DOI: 10.1016/j.fuel.2015.05.007. [11] 安江涛, 蒋勇, 邱榕, 等. CO2稀释及合成气构成对预混燃烧特性的影响 [J]. 燃烧科学与技术, 2011, 17(5): 437–442.AN Jiangtao, JIANG Yong, QIU Rong, et al. Effect of CO2-diluted oxygen and syngas composition on characteristics of premixed flame [J]. Journal of Combustion Science and Technology, 2011, 17(5): 437–442. [12] MOVILEANU C, GASA V, RAZUS D. Explosion of gaseous ethylene-air mixtures in closed cylindrical vessels with central ignition [J]. Journal of Hazardous Materials, 2012, 235-236(2): 108–115. DOI: 10.1016/j.jhazmat.2012.07.028. [13] RAZUS D, BRINZEA V, MITU M, et al. Explosion characteristics of LPG-air mixtures in closed vessels [J]. Journal of Hazardous Materials, 2009, 165(1): 1248–1252. DOI: 10.1016/j.jhazmat.2008.10.082. [14] RAZUS D, MOVILEANU C, OANCEA D. The rate of pressure rise of gaseous propylene-air explosions in spherical and cylindrical enclosures [J]. Journal of Hazardous Materials, 2007, 139(1): 1–8. DOI: 10.1016/j.jhazmat.2006.05.103. [15] PHYLAKTOU H N, ANDREWS G E, HERATH P. Fast flame speeds and rates of pressure rise in the initial period of gas explosions in large L/D cylindrical enclosures [J]. Journal of Loss Prevention in the Process Industries, 1990, 3(4): 355–364. DOI: 10.1016/0950-4230(90)80005-U. [16] 王颖. 20 L球形密闭装置内惰性气体抑制瓦斯爆炸实验研究 [D]. 太原: 中北大学, 2012: 22−23. [17] 贾宝山, 温海燕, 梁运涛, 等. 煤矿巷道内N2 及 CO2抑制瓦斯爆炸的机理特性[J]. 煤炭学报, 2013, 38(3): 361−366. DOI: 10.13225/j.cnki.jccs.2013.03.019.JIA Baoshan, WEN Haiyan, LIANG Yuntao, et al. Mechanism characteristics of CO2 and N2 inhibiting methane explosions in coal mine roadways [J]. Journal of China Coal Society, 2013, 38(3): 361−366. DOI: 10.13225/j.cnki.jccs.2013.03.019. [18] LI M A, YANG X, DENG J, et al. Effect of CO2 on explosion limits of flammable gases in goafs [J]. International Journal of Mining Science and Technology, 2010, 20(2): 193–197. DOI: 10.1016/S1674-5264(09)60183-6. [19] 余明高, 朱新娜, 裴蓓, 等. 二氧化碳-超细水雾抑制甲烷爆炸实验研究 [J]. 煤炭学报, 2015, 40(12): 2843–2848. DOI: 10.13225/j.cnki.jccs.2015.0068.YU Minggao, ZHU Xinna, PEI Bei, et al. Experimental study on methane explosion suppression using carbon dioxide and ultra-fine water mist [J]. Journal of Coal Science Engineering, 2015, 40(12): 2843–2848. DOI: 10.13225/j.cnki.jccs.2015.0068. [20] XIE Y, WANG J, CAI X, et al. Pressure history in the explosion of moist syngas/air mixtures [J]. Fuel, 2016, 185: 18–25. DOI: 10.1016/j.fuel.2016.07.072. [21] 张迎新, 吴强, 刘传海, 等. 惰性气体N2/CO2抑制瓦斯爆炸实验研究 [J]. 爆炸与冲击, 2017, 37(5): 906–912. DOI: 10.11883/1001-1455(2017)05-0906-07.ZHANG Yingxin, WU Qiang, LIU Chuanhai, et al. Experimental study on coal mine gas explosion suppression with inert gas N2/CO2 [J]. Explosion and Shock Waves, 2017, 37(5): 906–912. DOI: 10.11883/1001-1455(2017)05-0906-07. [22] SHANG R, ZHANG Y, ZHU M, et al. Laminar flame speed of CO2 and N2 diluted H2/CO/air flames [J]. International Journal of Hydrogen Energy, 2016, 41(33): 15056–15067. DOI: 10.1016/j.ijhydene.2016.05.064. [23] HAN M, AI Y, CHEN Z, et al. Laminar flame speeds of H2/CO with CO2 dilution at normal and elevated pressures and temperatures [J]. Fuel, 2015, 148: 32–38. DOI: 10.1016/j.fuel.2015.01.083. [24] CHEN Z, TANG C, FU J, et al. Experimental and numerical investigation on diluted DME flames: thermal and chemical kinetic effects on laminar flame speeds [J]. Fuel, 2012, 102(3): 567–573. DOI: 10.1016/j.fuel.2012.06.003. [25] AUNG K T, HASSAN M I, FAETH G M. Flame stretch interactions of laminar premixed hydrogen/air flames at normal temperature and pressure [J]. Combustion and Flame, 1997, 109(1/2): 1–24. DOI: 10.1016/S0010-2180(96)00151-4. [26] BRADLEY D, MITCHESON A. Mathematical solutions for explosions in spherical vessels [J]. Combustion and Flame, 1976, 26(2): 201–217. DOI: 10.1016/0010-2180(76)90072-9. [27] DAHOE A E, ZEVENBERGEN J F, LEMKOWITZ S M, et al. Dust explosions in spherical vessels: the role of flame thickness in the validity of the ‘cube-root law’ [J]. Journal of Loss Prevention in the Process Industries, 1996, 9(9): 33–44. DOI: 10.1016/0950-4230(95)00054-2. -






 下载:
下载: