Rheological properties of Composition B in slow cook-off process
-
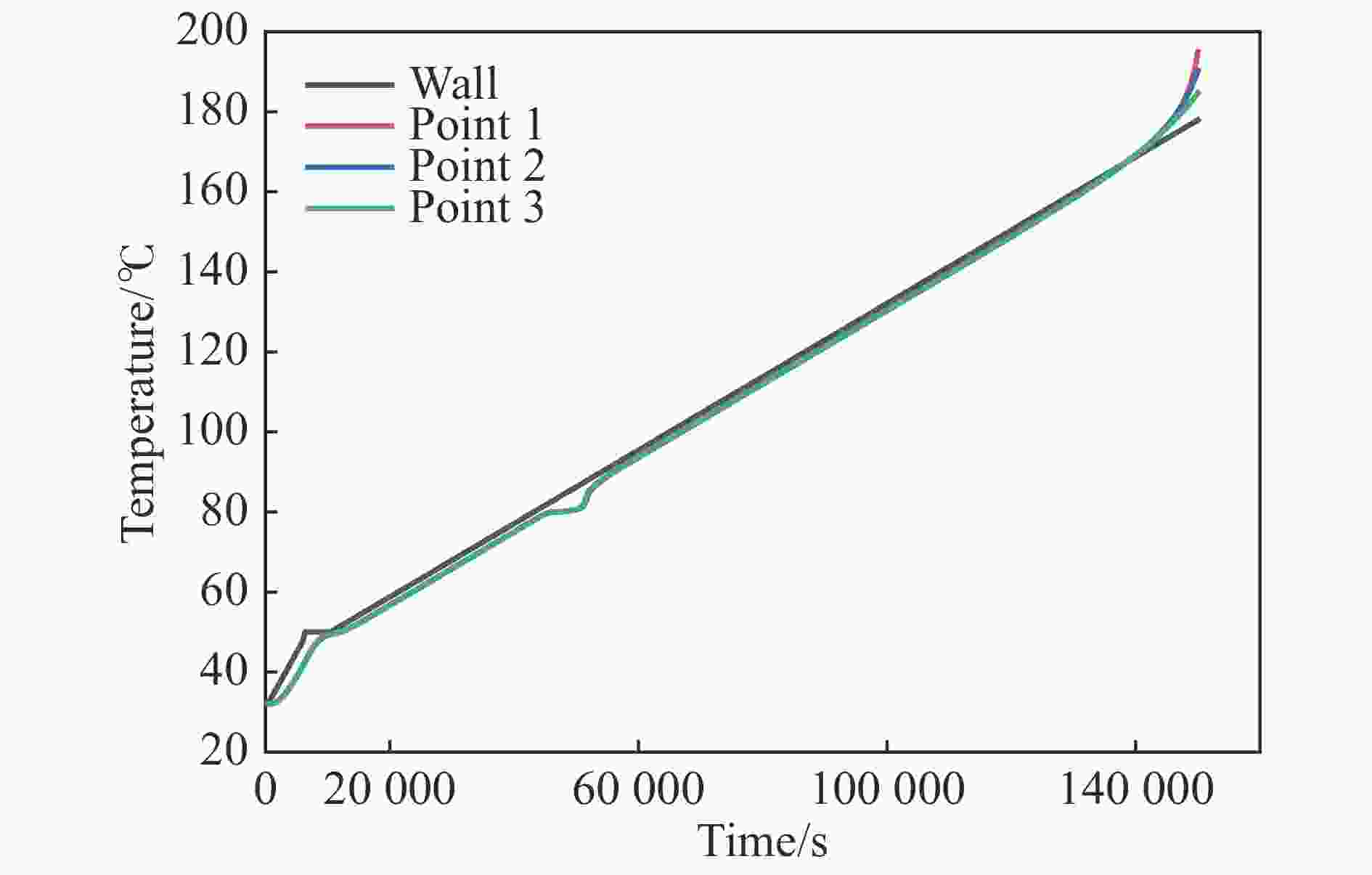
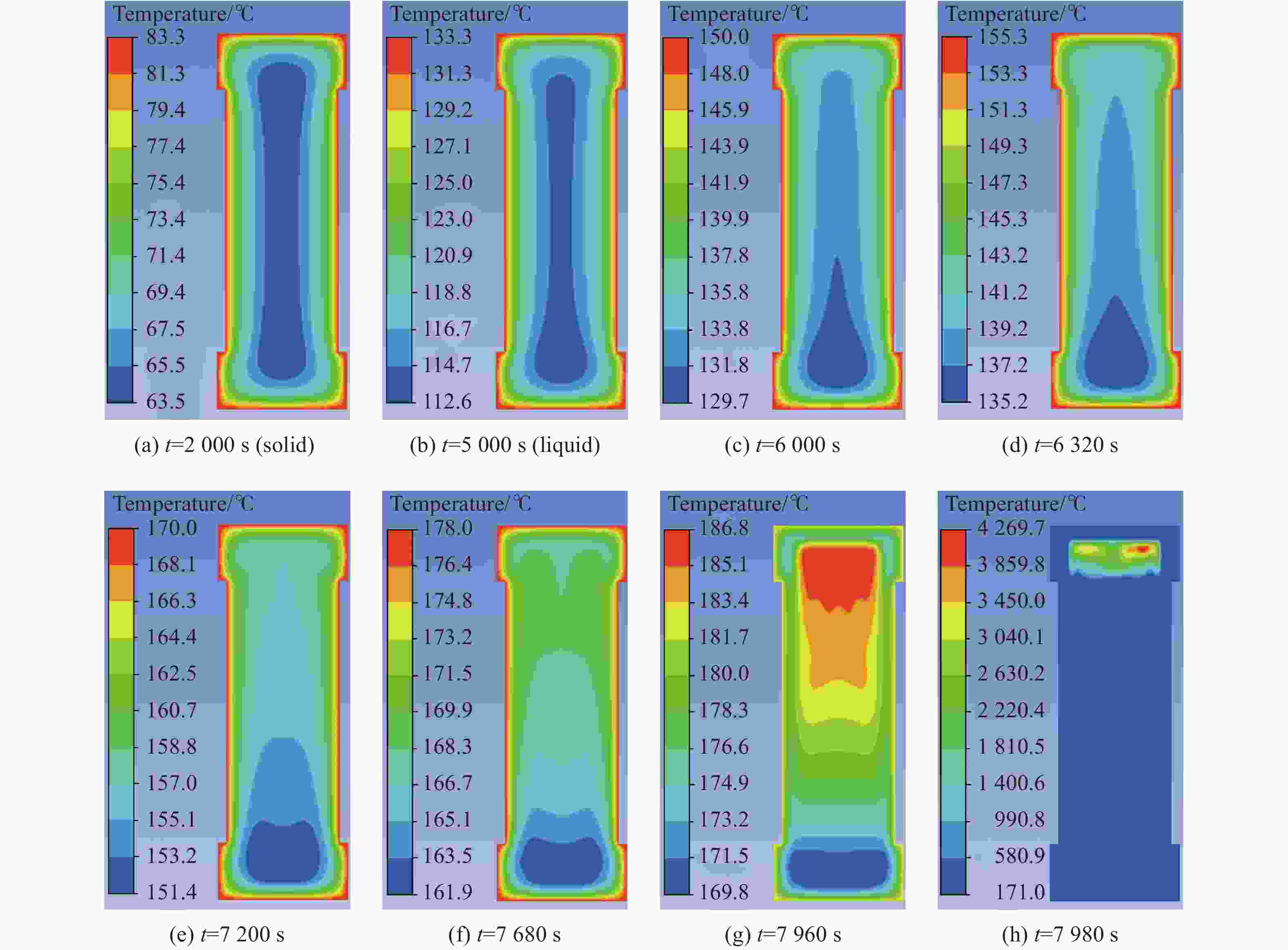
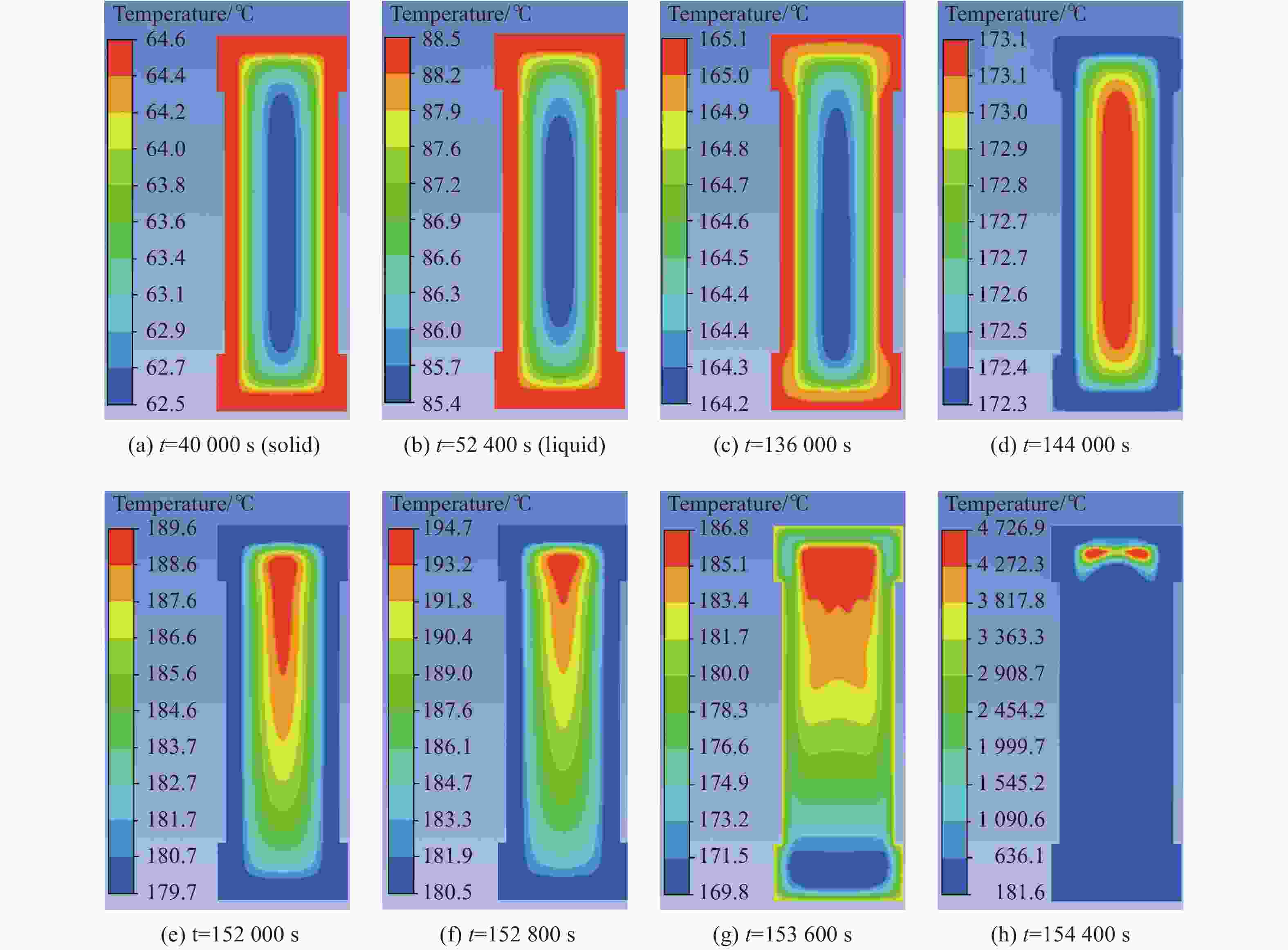
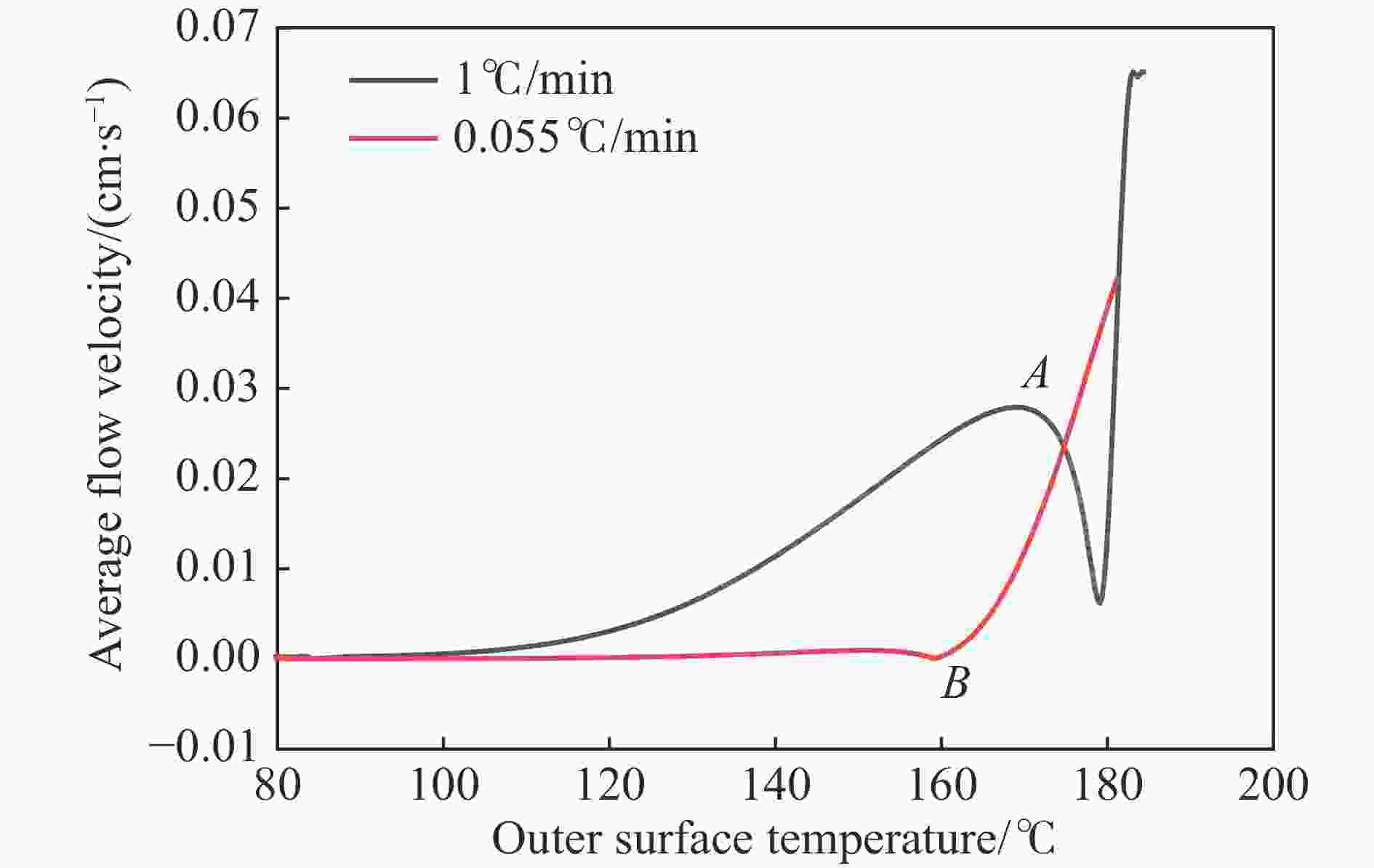
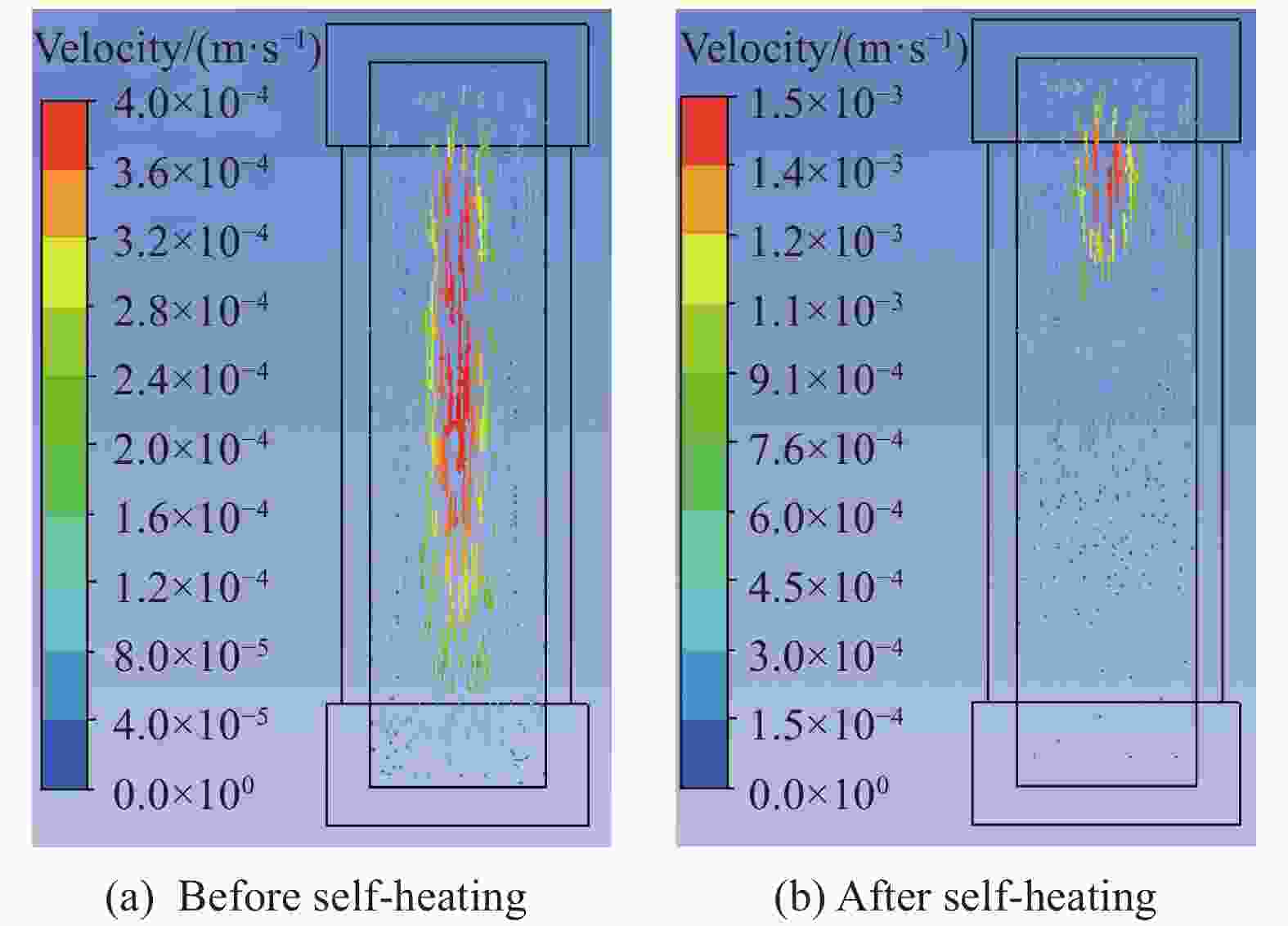
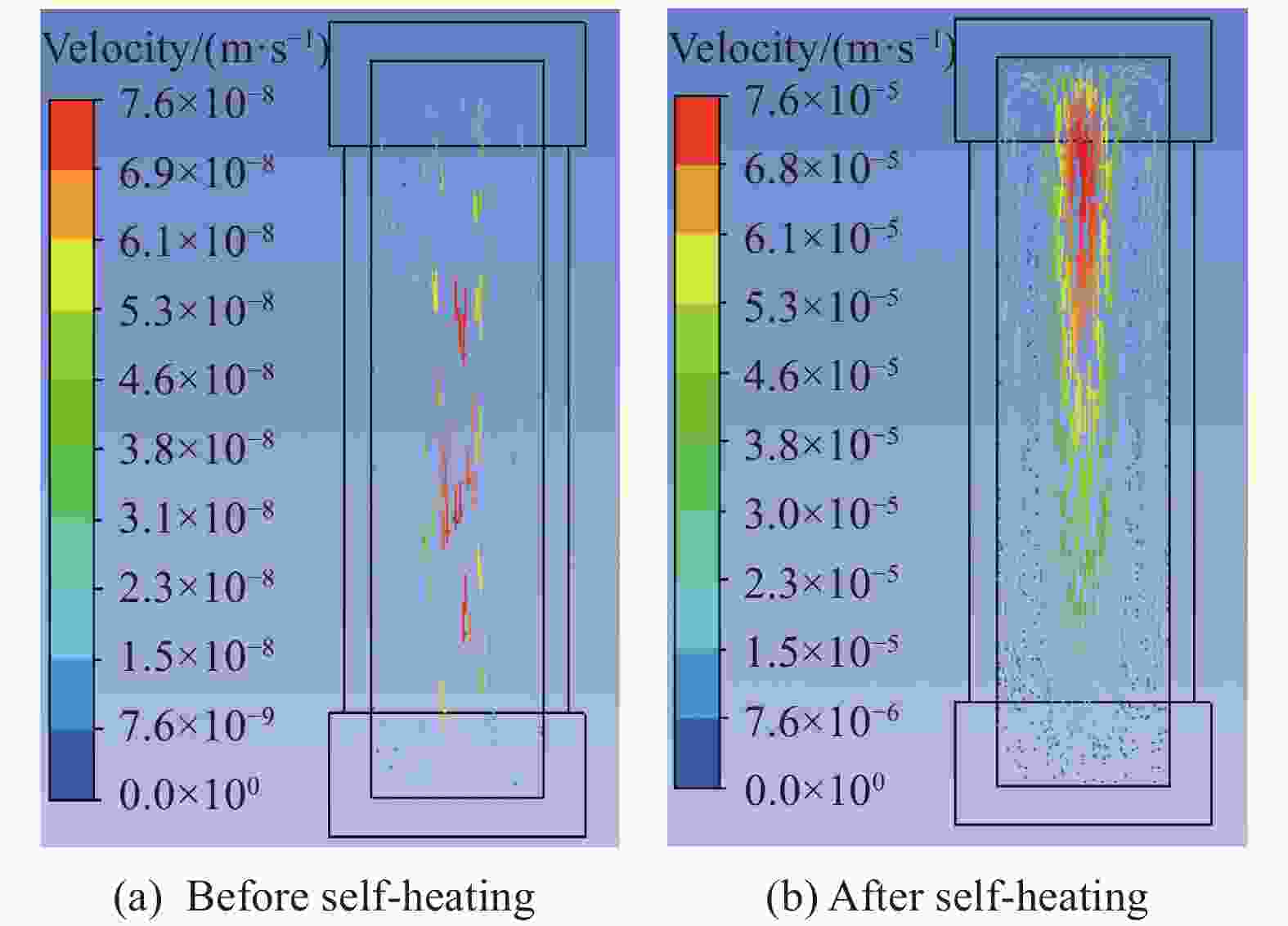
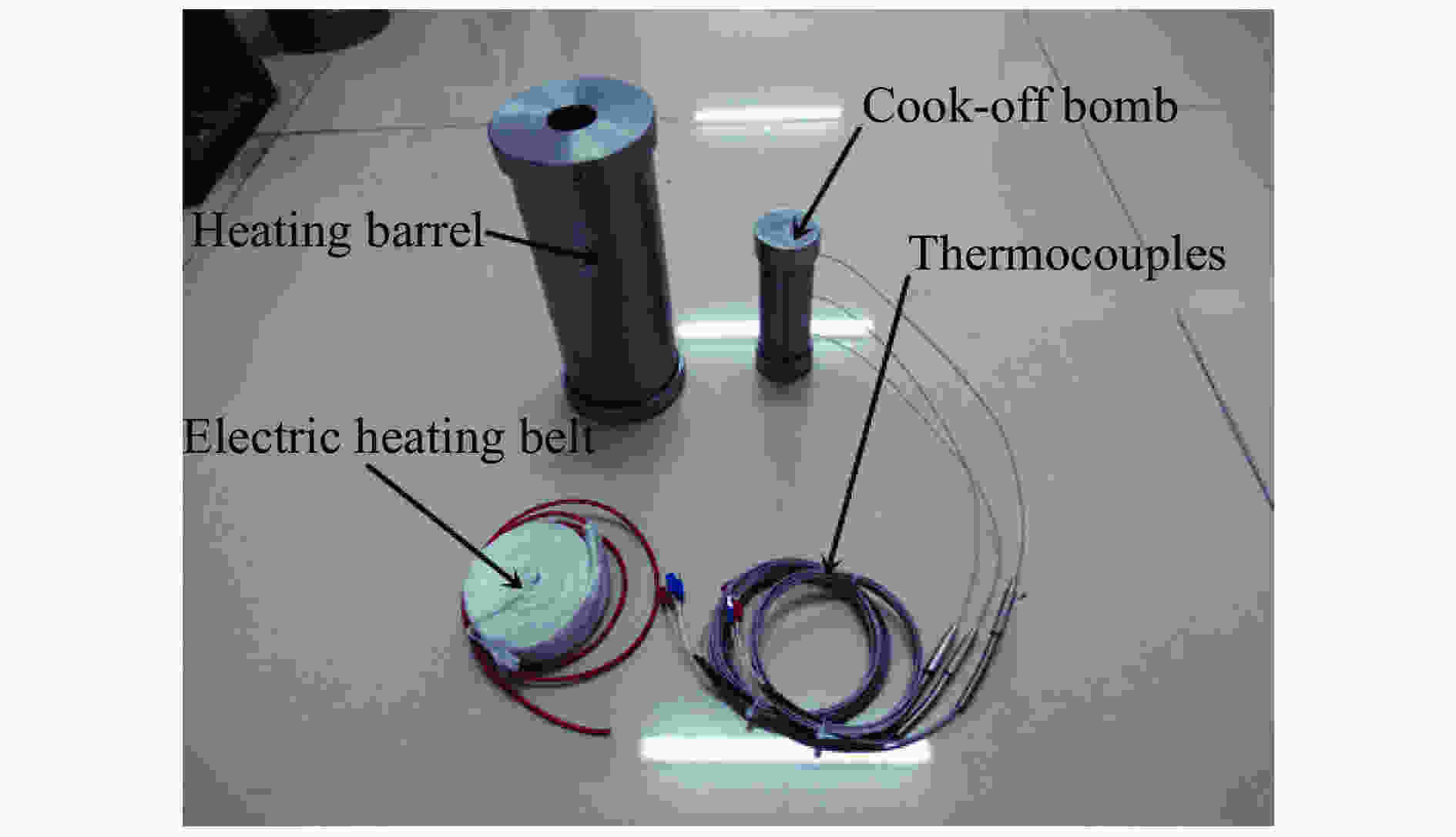
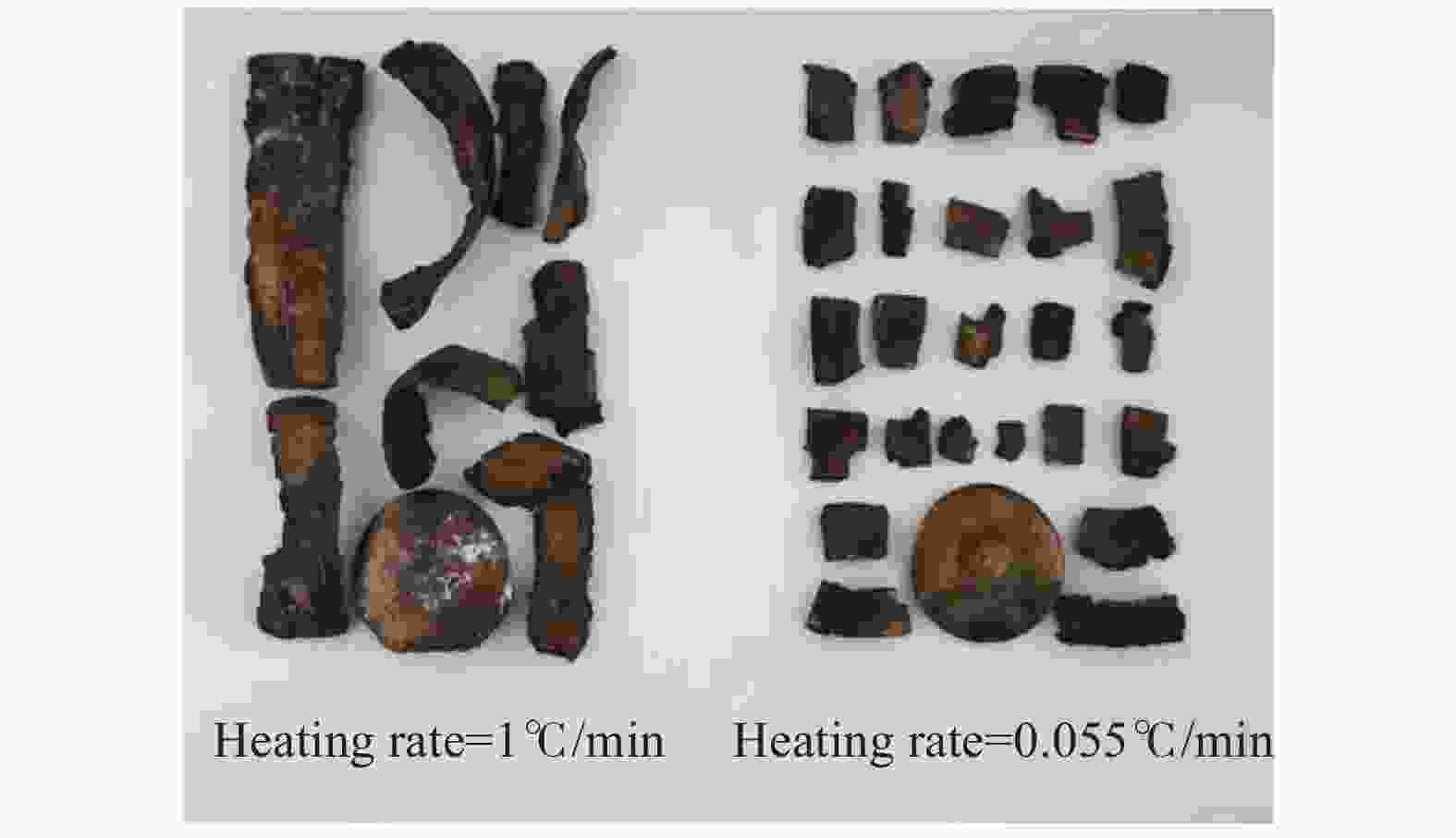
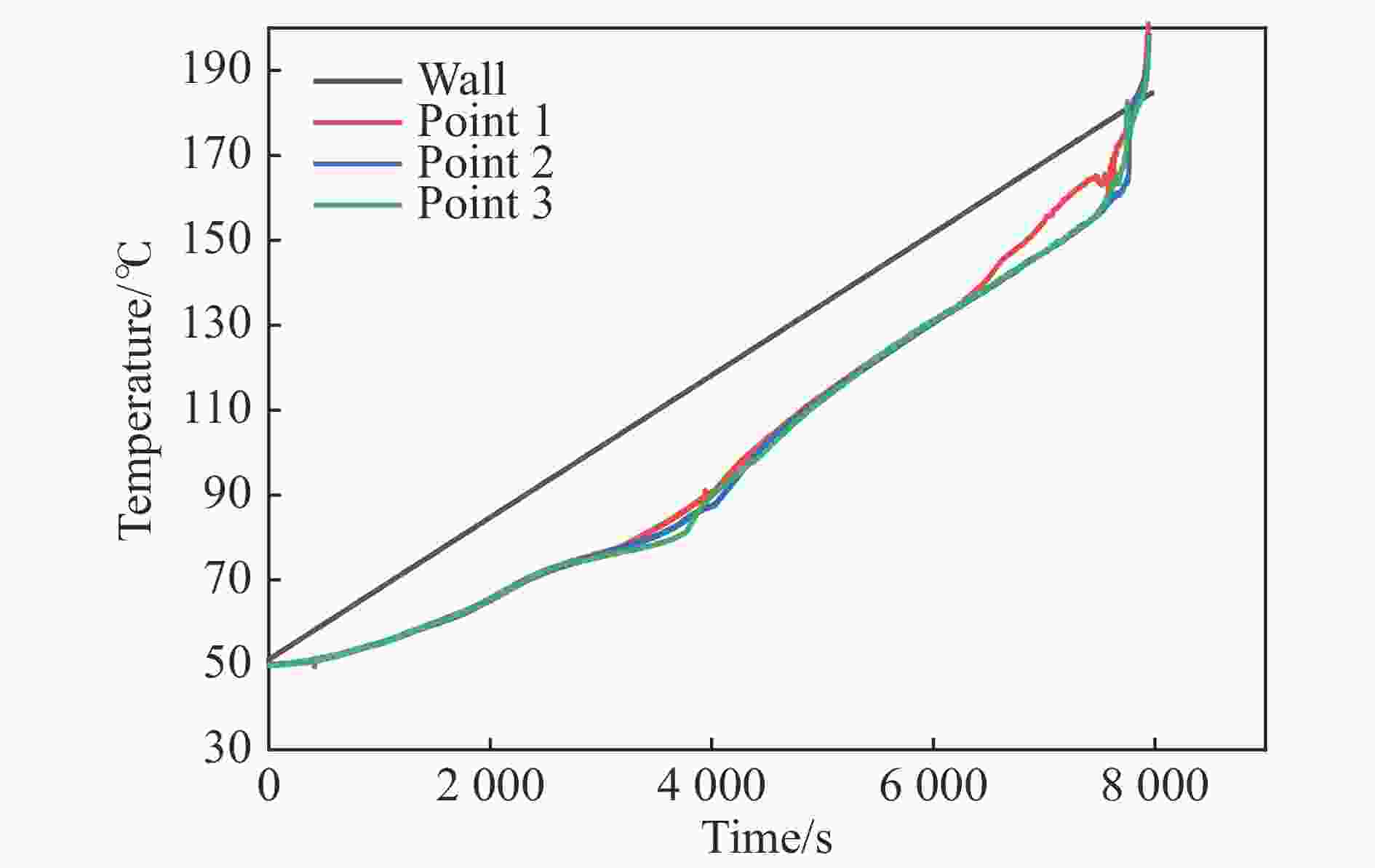
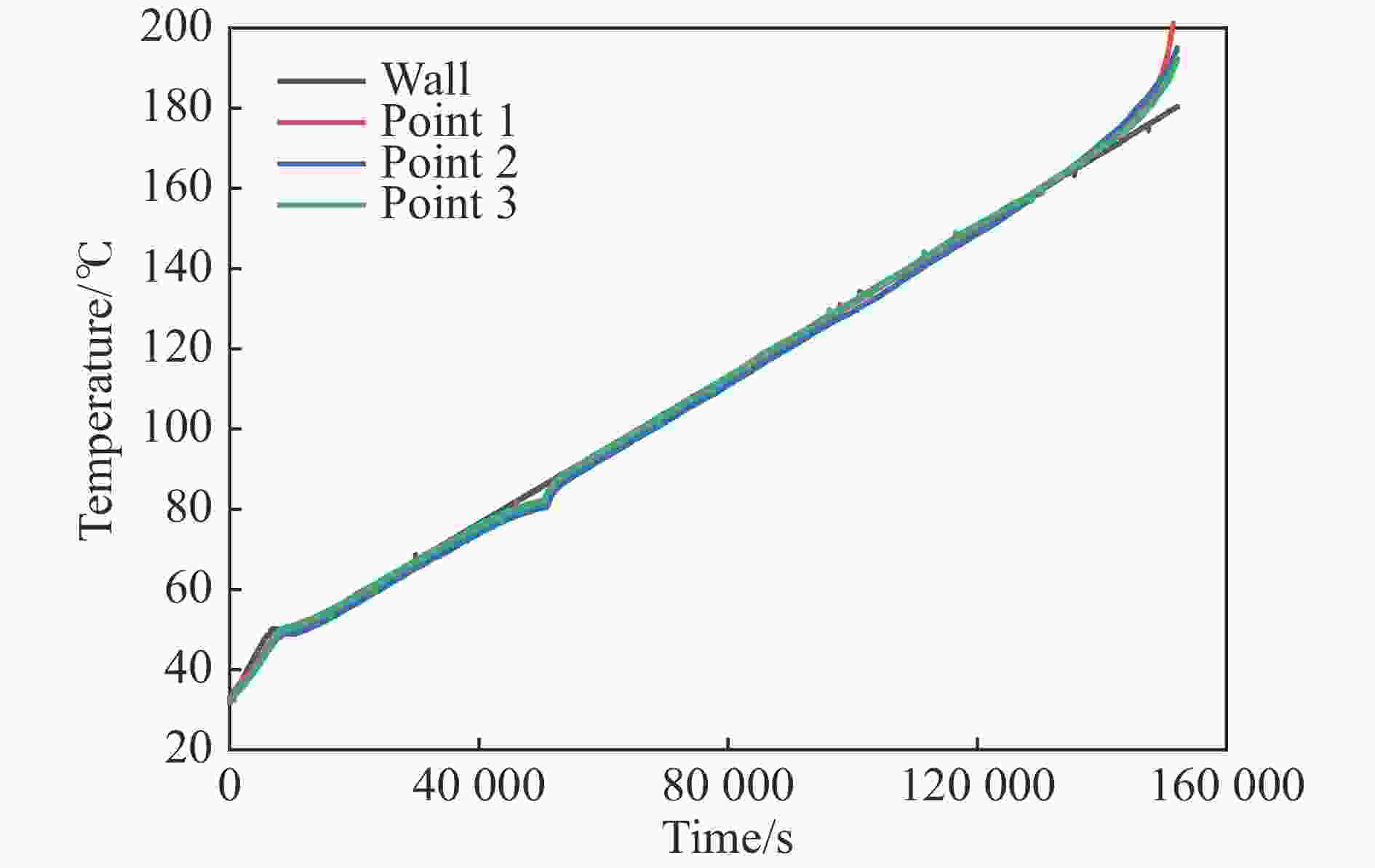
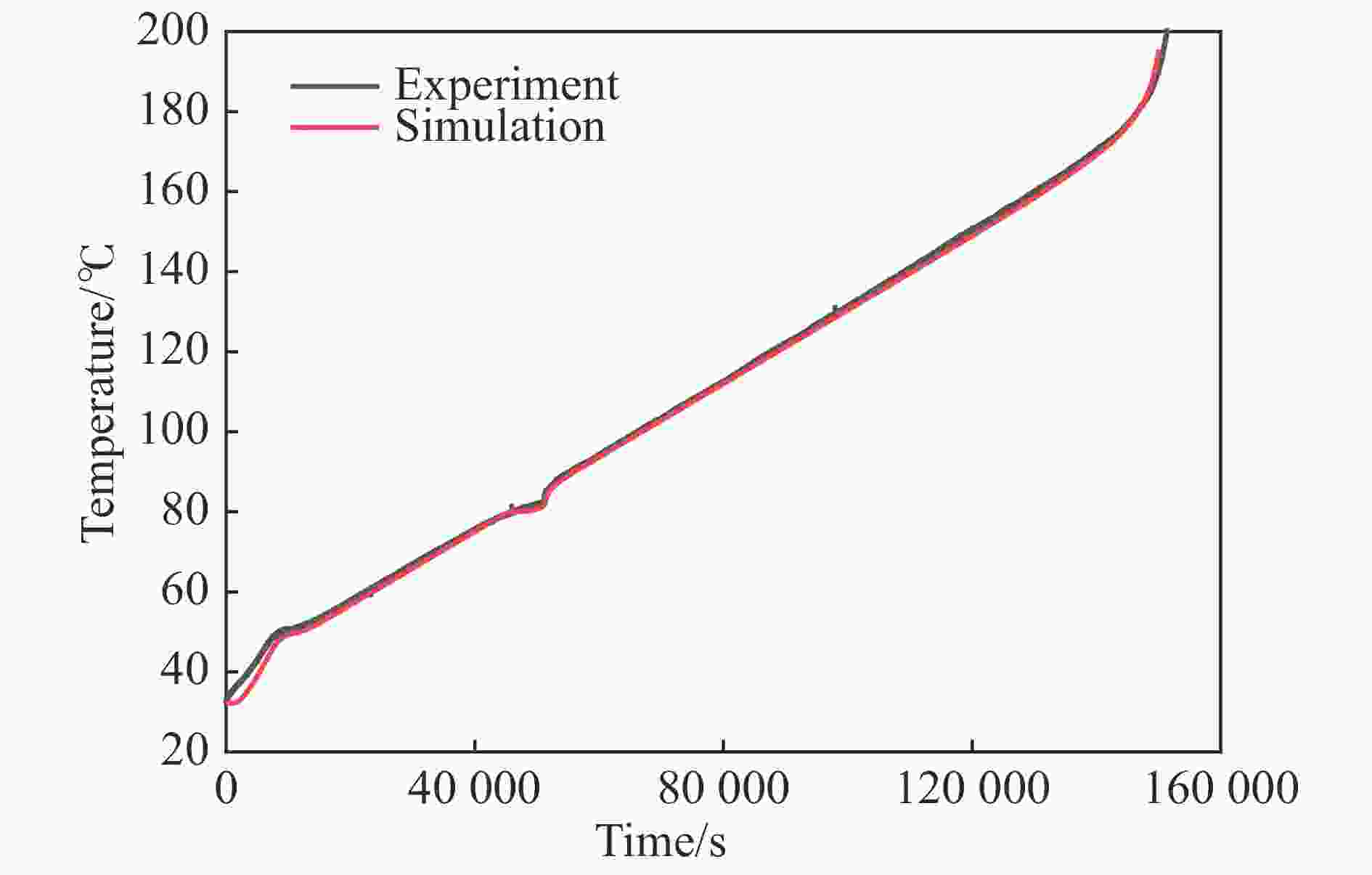
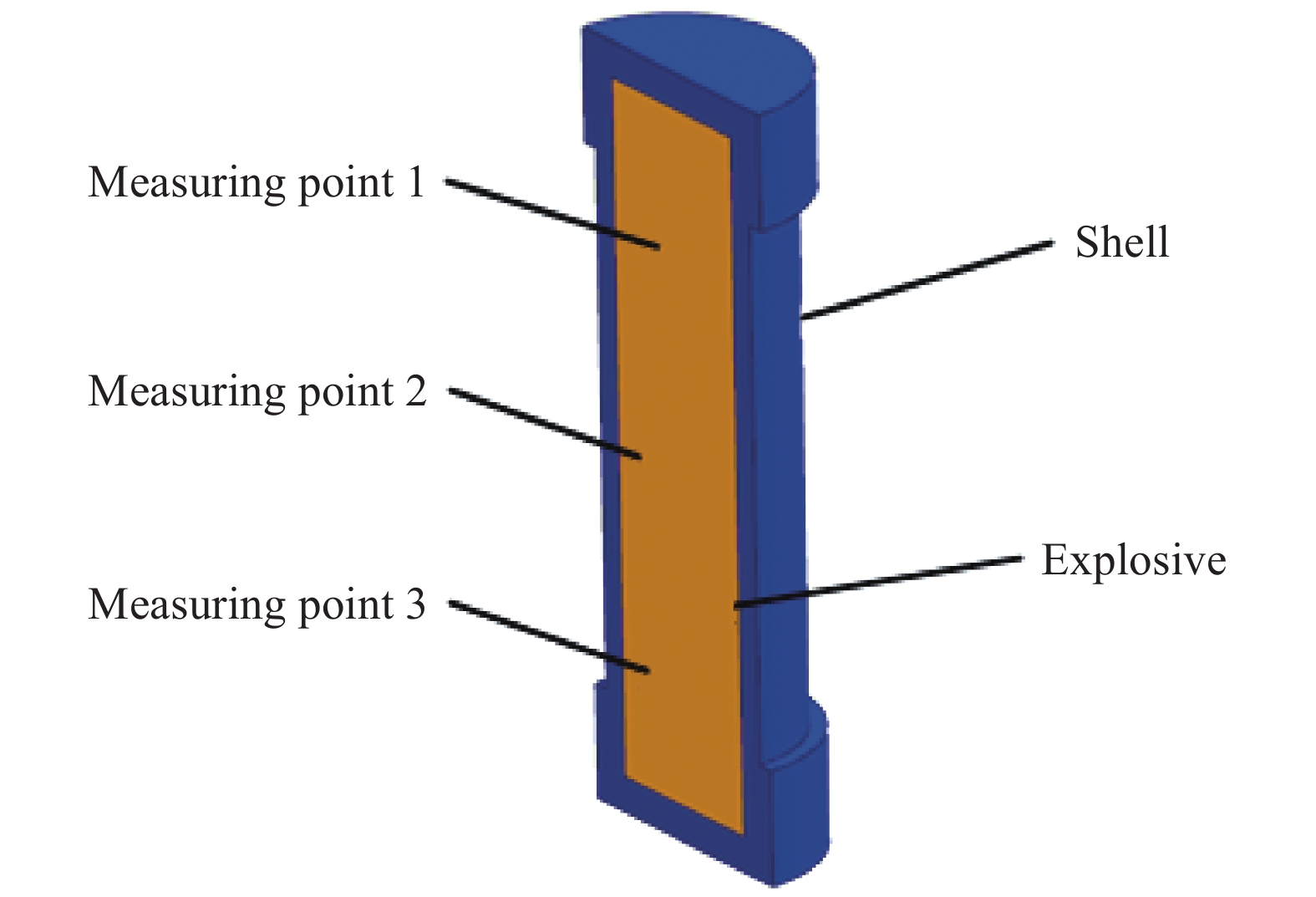
摘要: 为进一步探究熔铸炸药在烤燃过程中内部各物理场的变化情况,以B炸药为研究对象,完整地建立了基于Bingham流体模型的B炸药黏度计算模型并应用于慢速烤燃的数值模拟。通过数值模拟得到了B炸药在整个升温过程中上中下3个内部测点处的温度变化曲线并以烤燃试验加以验证,观察了弹体内部温度场与对流场的变化特点。结果表明:升温速率为1 ℃/min时,B炸药相变后逐渐开始流动,内部的温度场分布也随之改变,炸药出现自热反应与最终响应的区域都在弹体上部;升温速率为0.055 ℃/min时,炸药相变后内部很长时间内仍表现出类固相温度场的分布特点,当炸药出现自热反应后,才逐渐开始流动,温度场也逐渐转变为典型的液相温度场,炸药最终响应点在弹体上部,但最早出现自热反应的区域在弹体中心。Abstract: In order to investigate the changes of the internal physical fields of melt-castable explosives in cook-off, Composition B was chosen as the object. A complete viscosity model of Composition B based on the Bingham flow model was first established, and then applied in the numerical simulations of slow cook-off. In this way, the temperature curves of three inner measuring pointsthat located in the upper, middle and lower respectively were obtained and further testified with cook-off experimental measurements. Moreover, the variation of the inner temperature field in the whole process was observed as well. The results showed that when the heating rate was 1 ℃/min, the viscosity flow of Composition B appeared soon after the phase change, and the inner temperature field changed with that. The self-heating and ignition occurred in the upside area of the shell. But when the heating rate was 0.055 ℃/min, the inner temperature field was still like a solid phase after the phase change was completely done for a long time, and the viscosity flow appeared after the self-heating started, the inner temperature field gradually began to change like a liquid phase just at that time. The ignition area was in the upside of the shell too, but the self-heating area was in the middle of the shell. The contradictory points of view in previous studies can be preliminarily explained by this model.
-
Key words:
- slow cook-off test /
- melt-castable explosives /
- viscosity /
- Bingham fluid /
- responses
-
表 1 屈服应力阈值计算参数
Table 1. Parameters to calculate the yield stress threshold
φc C/Pa n 0.336 6 874 2.37 表 2 φmax计算参数
Table 2. Calculating parameters of φmax
φ0 φ∞ Z m 0.55 0.713 8.73 0.398 表 3 φ计算参数
Table 3. Calculating parameters of φ
φa A B C D Tm,RDX/℃ 0.6 0.042 6 0.075 3 0.134 0.747 4 204 表 4 壳体材料参数
Table 4. Material parameters for the shell
材料 密度/(kg∙m−3) 比热容/(J∙kg−1) 导热系数/(W∙m−1∙K−1) 45#钢 7 850 475 15 表 5 B炸药物性参数
Table 5. Physical parameters of Comp B
密度/(kg∙m−3) 比热容/(J∙kg−1) 导热系数/(W∙m−1∙K−1) 熔化热/(kJ∙kg−1) 相变起始温度Ts/℃ 相变结束温度T1/℃ $\begin{array}{*{20}{c}} {1\;690}&{T {\simfont\text{≤}} {T_s}} \\ {1\;690 - 0.675\left( {T - {T_{\rm{s}}}} \right)}&{T {\simfont\text{>}} {T_{\rm{s}}}} \end{array}$ 1 126 $\begin{array}{*{20}{c}} {0.17}&{T {\simfont\text{<}} {T_{\rm{s}}}} \\ {0.17 - 0.025\left( {T - {T_{\rm{m}}}} \right)}&{{T_{\rm{s}}} {\simfont\text{≤}} T {\simfont\text{≤}} {T_{\rm{l}}}} \\ {0.15}&{T {\simfont\text{>}} {T_{\rm{l}}}} \end{array}$ 128 80 82 表 6 B炸药化学反应动力学参数
Table 6. Chemical kinetic parameters of Comp B
活化能/(kJ∙mol−1) 反应热/(J∙kg−1) 指前因子 22 5.82×106 2.01×1018 -
[1] 智小琦. 弹箭炸药装药技术 [M]. 北京: 兵器工业出版社, 2012: 7−8.ZHI X Q. Charge technology of explosive on projectile and rocket [M]. Beijing: Ordnance Industry Press, 2012: 7−8. [2] HOBBS M L, KANESHIGE M J, ERIKSON W W. Predicting large-scale effects during cookoff of PBXs and melt-castable explosives [C] // Proceedings of the 26th International Colloquium on the Dynamics of Explosions and Reactive Systems. Boston: MA: OSTI, 2017: 152−158. [3] MAIENSCHEIN J L, McCLELLAND M A, WARDELL J F, et al. ALE3D model predictions and experimental analysis of the cookoff response of Comp B [C] // Proceedings of Joint Army Navy NASA Air Force (JANNAF) Meeting. Colorado Springs, CO, USA: LLNL, 2003: 51−64. [4] GLASCOE E A, DEHAVEN M R, MCCLELLAND M, et al. Mechanisms of Comp-B thermal explosions [C] // Proceedings of the 15th International Detonation Symposium. San Francisco: LLNL, 2014: 376−385. [5] NICHOLS A L, SCHOFIELD S. Modeling the response of fluid/melt explosives to slow cook-off [C] // Proceedings of the 15th International Detonation Symposium. San Francisco: LLNL, 2014: 1128−1136. [6] FEDOROFF B T, SHEFFIELD O E, KAYE S M. Encyclopedia of explosives and related items [M]. Dover, NJ: Picatinny Arsenal, 1962. [7] HOBBS M L, KANESHIGE M J, ANDERSON M U. Cookoff of a melt-castable explosive (COMP-B) [C] // Proceedings of the 27th Propulsion Systems Hazards Joint Subcommittee Meeting. Monterrey: SNL-NM, 2012: 1020−1034. [8] SANHYE W, DUBOIS C, LAROCHE I, et al. Numerical modeling of the cooling cycle and associated thermal stresses in a melt explosive charge [J]. AIChE Journal, 2016, 62(10): 3797–3811. DOI: 10.1002/aic.15288. [9] NUNEZ M P, ZERKLE D K, ZUCKER J M. The rheology of molten Composition B [R]. NM: Los Alamos, 2012. [10] ZERKLE D K, NUNEZ M P, ZUCKER J M. Molten composition B viscosity at elevated temperature [J]. Journal of Energetic Materials, 2016, 34(4): 368–383. DOI: 10.1080/07370652.2015.1102179. [11] DAVIS S M, ZERKLE D K, SMILOWITZ L B, et al. Integrated rheology model: explosive composition B-3 [J]. Journal of Energetic Materials, 2018, 36(4): 398–411. DOI: 10.1080/07370652.2018.1451573. [12] DAVIS S M, ZERKLE D K. Short communication: estimation of yield stress/viscosity of molten Octol [J]. AIP Advances, 2018, 8(5): 055202. [13] MACOSKO C W. Rheology principles, measurements and applications [M]. New York: VCH Publishers, 1994: 92−98. [14] MORRISON F A. Understanding rheology [M]. New York: Oxford University Press, 2001: 232. [15] QUEMADA D. Rheology of concentrated disperse systems and minimum energy dissipation principle: I. viscosity-concentration relationship [J]. Rheologica Acta, 1977, 16(1): 82–94. DOI: 10.1007/BF01516932. [16] ZHOU J Z Q, UHLHERR P H, LUO F T. Yield stress and maximum packing fraction of concentrated suspensions [J]. Rheologica Acta, 1995, 34(6): 544–561. DOI: 10.1007/BF00712315. [17] Fluent Inc. FLUENT user’s guide [M]. US: Fluent Inc, 2006. [18] MCCLELLAND M A, GLASCOE E A, NICHOLS A L, et al. ALE3D simulation of incompressible flow, heat transfer, and chemical decomposition of Comp B in slow cookoff experiments [C] // Proceedings of International Detonation Symposium. San Francisco: LLNL, 2014: 517−528. -







 下载:
下载: