Influence of ignition criterion and dilution gas on ignition delay of ethylene
-
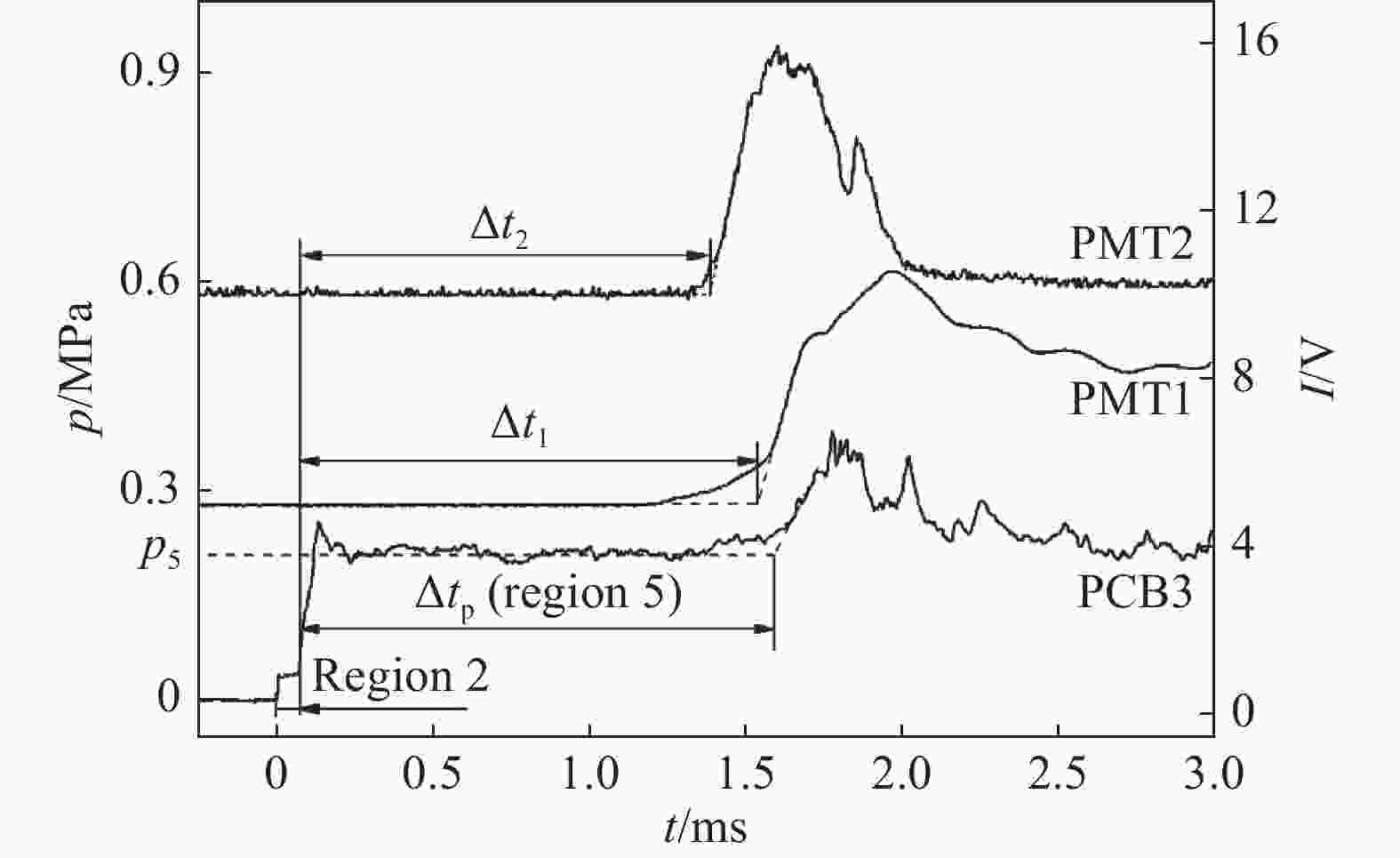
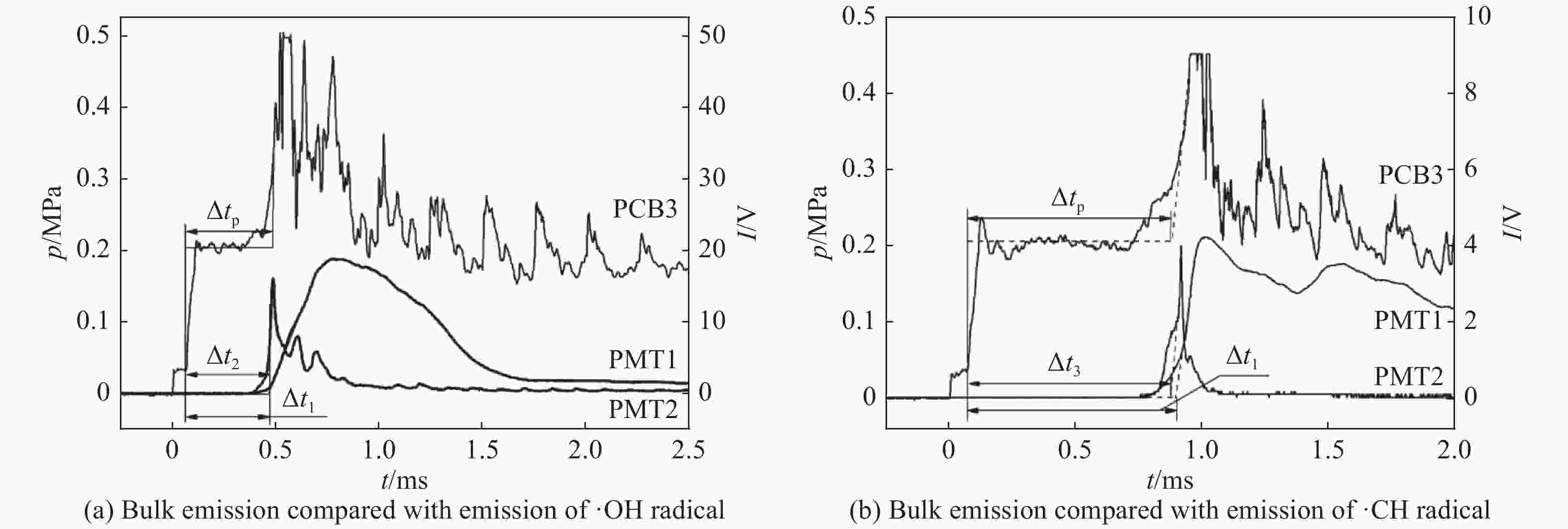
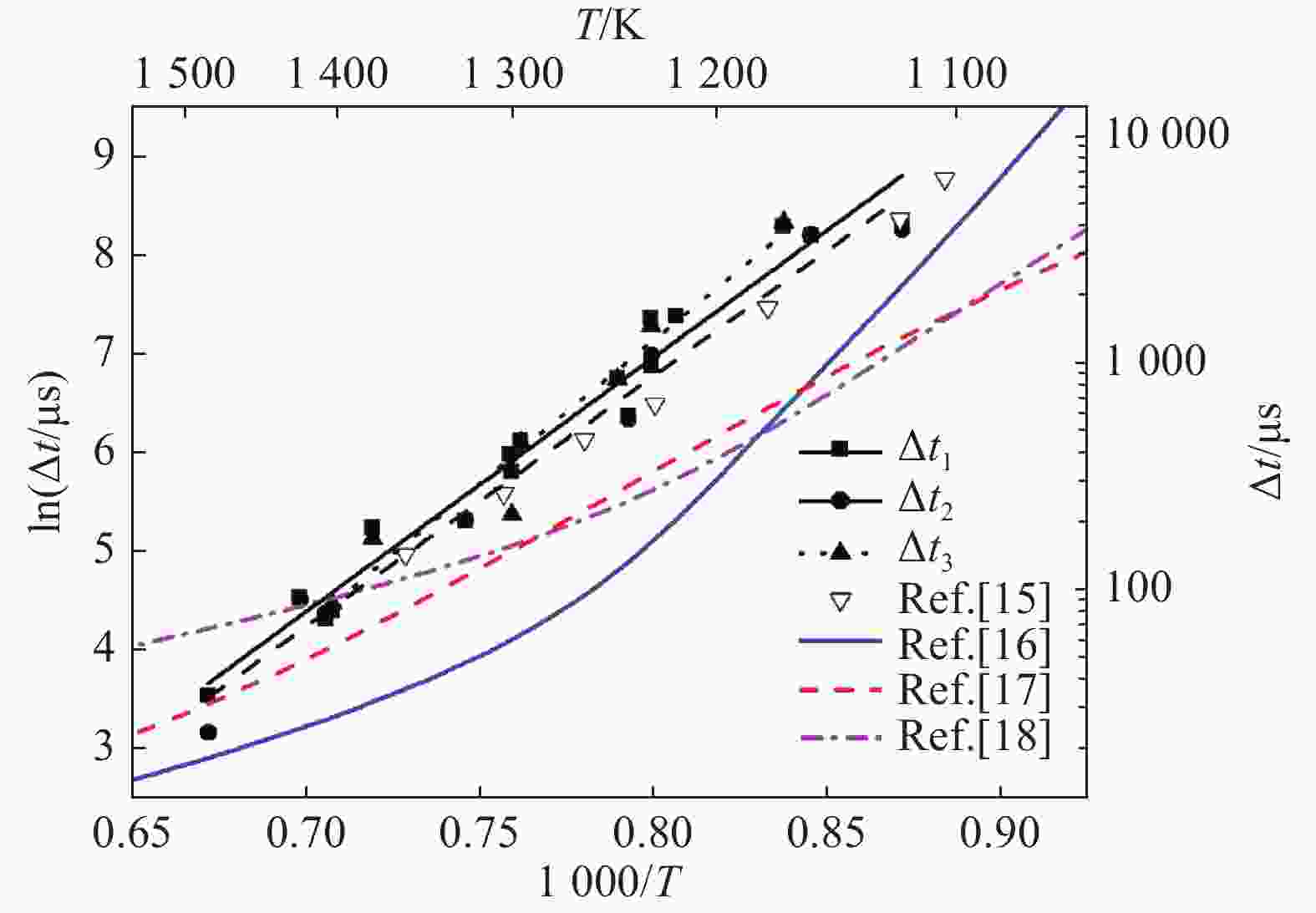
摘要: 利用矩形截面激波管研究点火准则和稀释气体对乙烯点火延时的影响。采用压电传感器记录测点压力时间历程,采用光谱仪和光电倍增管记录自发光强时间历程,以压力、总自发光强与·OH和·CH自由基特定能级发射光强等信号判定是否发生自点火,给出自点火过程的时间起始点和终止点,得到了不同点火准则和稀释气体对应的乙烯/氧气/氮气和乙烯/氧气/氩气点火延时。结果表明:相同工况的乙烯点火延时测量数据相对误差约为15%,数据验证了本文实验和测量方法可靠性。针对当量比为1.0、压力为0.2 MPa,得到了温度范围为905~1 489 K,稀释气体的摩尔分数为75%氮气和75%氩气时的乙烯点火延时,给出点火延时和温度拟合的Arrhenius型表达式。不同点火准则会影响所测点火延时数据,但多次测量结果确定的点火延时和温度变化规律近似相同。不同稀释气体对激波管自点火流场的影响表现为和流场均匀性以及混合物比热相关。相同工况的乙烯/氧气/氮气点火延时大于乙烯/氧气/氩气点火延时。高温区和低温区的乙烯/氧气/氩气点火延时与温度的拟合关系不同,转折温度约为1 121 K。Abstract: Ignition delay of ethylene (C2H4) are measured under different temperatures in a rectangle shock tube to recognize the effects from diluent gases (nitrogen or argon) and criteria which is identified by pressure, bulk and radical chemiluminescences of OH and CH at specified wavelengths. Pressures were recorded by piezoelectric sensors (PCBs), and bulk chemiluminescence was detected by a photomultiplier (PMT) and an optical fiber. The chemiluminescences of OH and CH radicals were grated by spectrometer first, and then recorded by the PMT. The ignition delay is determined from the pressure and intensity histories of bulk and radical chemiluminescences at the points which share the same distances from the close end. Ignition delay database was built for mixture of C2H4/O2/N2 and C2H4/O2/Ar. Measurement and methodology are verified by the repeated experimental data under the same conditions. In the case of stoichimetric equivalence and pressure at 0.2 MPa, ignition delays were obtained and fitted with temperature as Arrhenius formula for mixtures of C2H4/O2/N2 and C2H4/O2/Ar at temperature ranging from 905 K to 1 489 K. Results show that the relative error of ignition delays is about 15%. Based on pressure, bulk and radical chemiluminescences, the relationships between the ignition delay and temperature remain the same although the ignition delay from single measurement is a bit different. Basically, the ignition delay of C2H4/O2/N2 is greater than that of C2H4/O2/Ar. The fitting relationship between ignition delay and temperature of C2H4/O2 /Ar in high temperature zone and low temperature zone is different, and the turning temperature is about 1 121 K.
-
Key words:
- ignition delay /
- shock tube /
- ignition criteria /
- dilute gas /
- ethylene
-
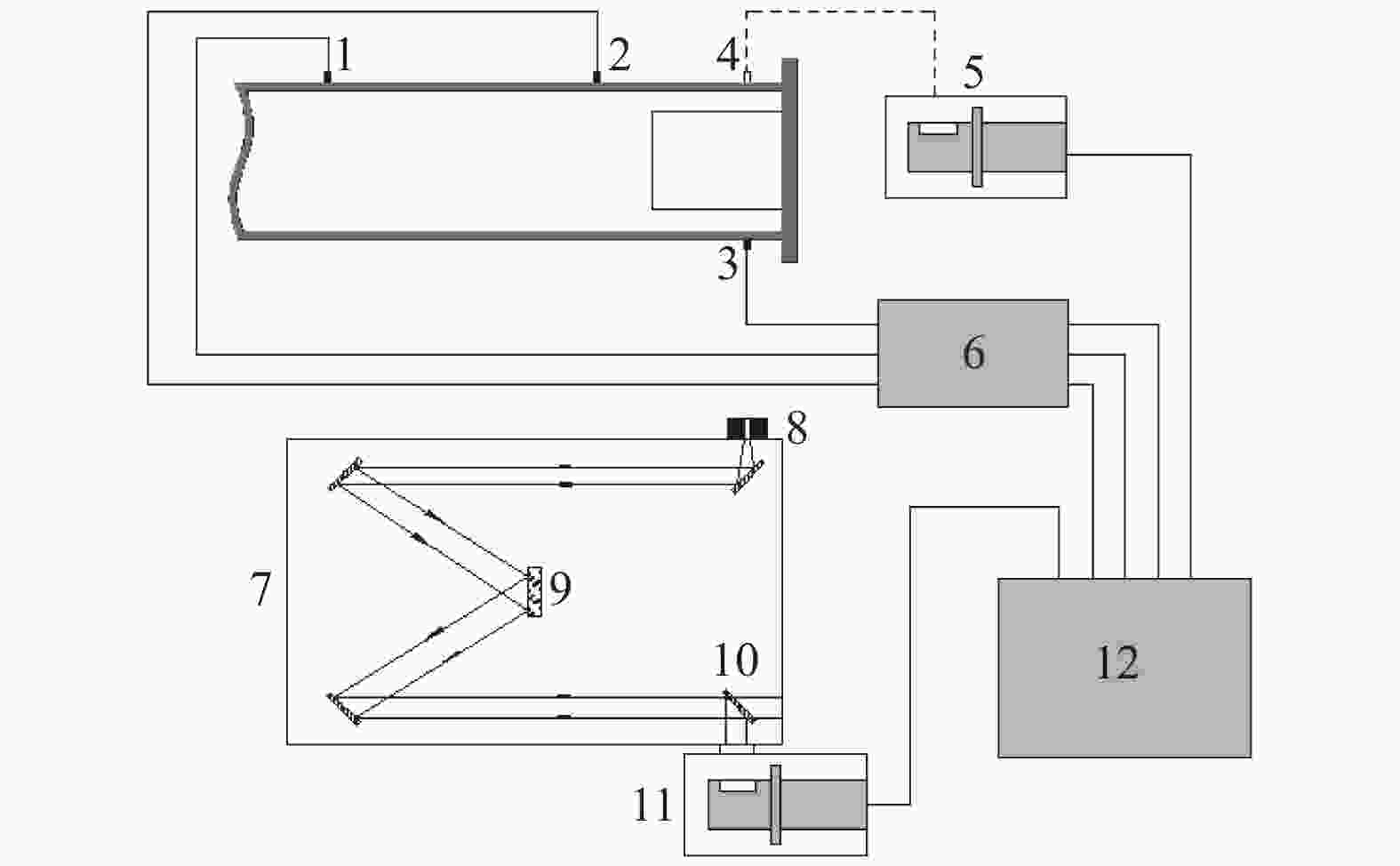
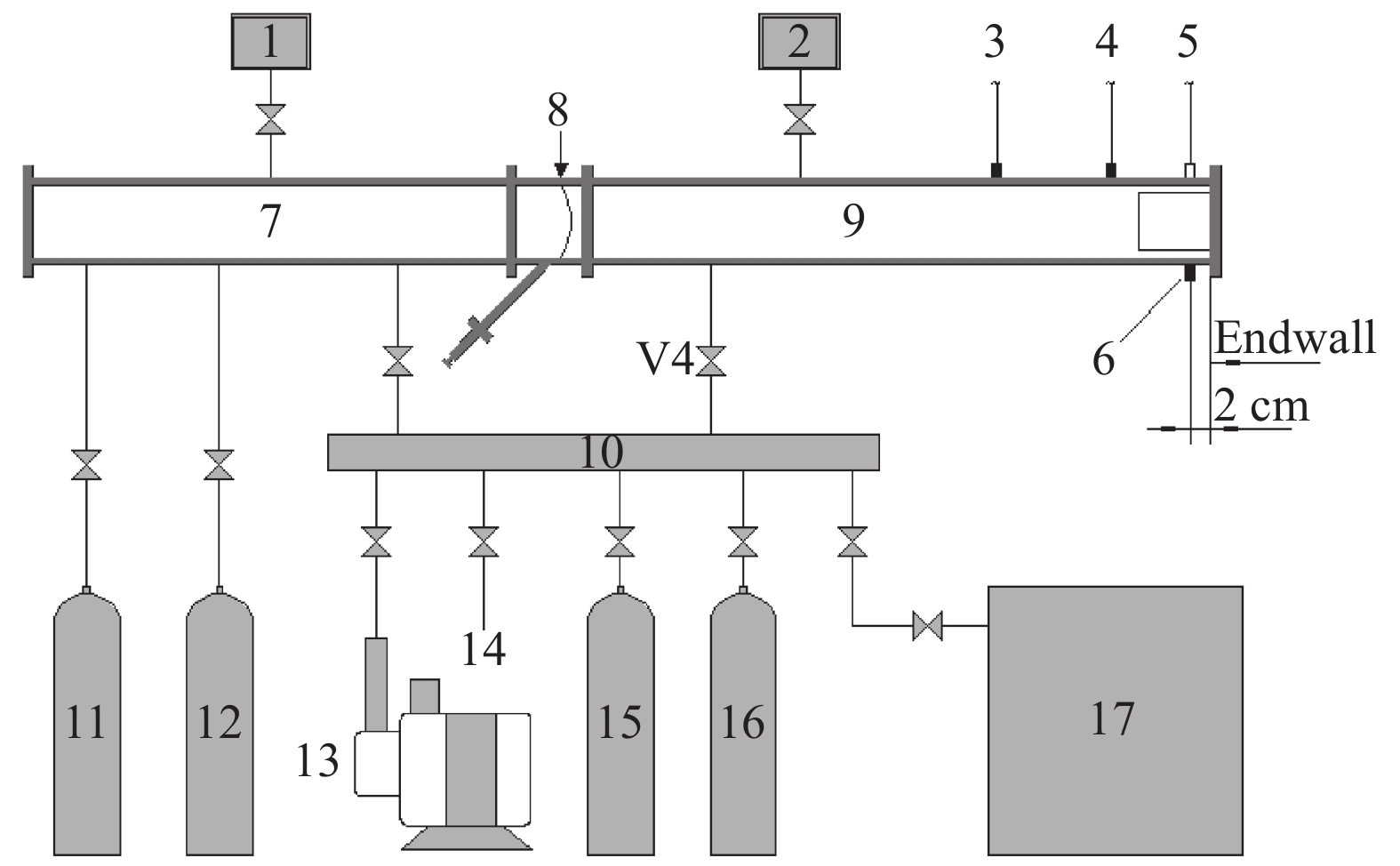
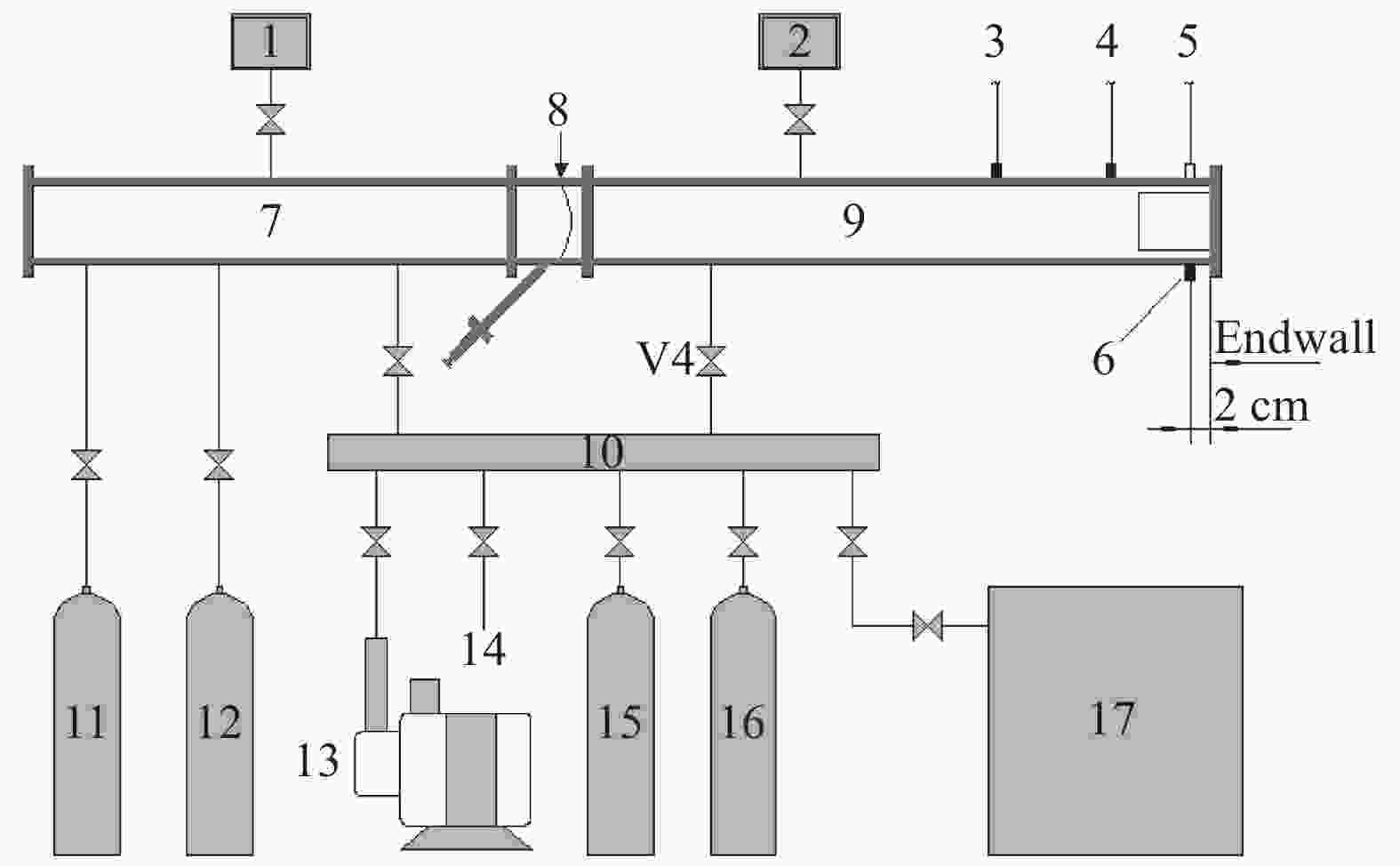
图 1 激波管和气路系统示意图
1. Pressure gauge; 2. Vacuum gauge; 3. PCB1; 4. PCB2; 5. Fiber; 6. PCB3; 7. Driver section; 8. Diaphragm section; 9. Driven section; 10. Gas distribution section; 11. He; 12. N2; 13. Vacuum pump; 14. Exhausting exit; 15. C2H4; 16. Dilute gas; 17. Premixing tank
Figure 1. Sketch of shock tube and gas distribution
表 1 乙烯/氧气/氩气点火延时重复性
Table 1. Repeatability of ignition delay of C2H4/O2/Ar
实验 us/(m·s−1) Ms p5/MPa T5/K Δt1/μs 1 802.8 2.510 0.192 1 321 427 2 799.6 2.503 0.192 1 311 390 3 800.3 2.506 0.191 1 313 439 4 805.8 2.523 0.194 1 328 337 5 803.4 2.508 0.191 1 323 382 6 796.6 2.479 0.188 1 304 383 7 813.1 2.530 0.195 1 352 325 平均值 803.1±10.0 2.508±0.029 0.192±0.004 1 322±30 383±58 -
[1] KUO K K. Principles of combustion [M]. New York: John Wiley & Sons Inc., 1986. [2] HANSON R K, DAVIDSON D F. Recent advances in laser absorption and shock tube methods for studies of combustion chemistry [J]. Progress in Energy and Combustion Science, 2014, 44(5): 103–114. [3] BARARI G, PRYOR O, KOROGLU B, et al. High temperature shock tube experiments and kinetic modeling study of diisopropyl ketone ignition and pyrolysis [J]. Combustion and Flame, 2017, 177: 207–218. DOI: 10.1016/j.combustflame.2016.12.003. [4] REN W, DAVIDSON D F, HANSON R K. IR laser absorption diagnostic for C2H4 in shock tube kinetics studies [J]. International Journal of Chemical Kinetics, 2012, 44(6): 423. DOI: 10.1002/kin.20599. [5] ROOSE T R, HANSON R K, KRUGER C H. A shock tube study of the decomposition of NO in the presence of NH3 [C] // Symposium (International) on Combustion, 1981, 18(1): 853-862. [6] 胡弘浩. 乙烯点火特性及其污染效应的激波管研究[D]. 重庆: 重庆大学, 2012.HU H H. A shock tube study of ignition delay characteristics of ethylene and contamination effect [D]. Chongqing: Chongqing University, 2012. [7] BAKER J A, SKINNER G B. Shock-tube studies on the ignition of ethylene-oxygen-argon mixtures [J]. Combustion and Flame, 1972, 19(3): 347–350. DOI: 10.1016/0010-2180(72)90004-1. [8] SUZUKI M, MORIWAKI T, OKAZAKI S, et al. Oxidation of ethylene in shock tube [J]. Astronautica Acta, 1973, 18(5): 359–365. [9] BROWN C J, THOMAS G O. Experimental studies of shock-induced ignition and transition to detonation in ethylene and propane mixtures [J]. Combustion and Flame, 1999, 117(4): 861–870. DOI: 10.1016/S0010-2180(98)00133-3. [10] CADMAN P, BAMBREY R J, BOX S K, et al. Ethylene combustion studied over a wide temperature range in high-temperature shock waves [J]. Combustion Science and Technology, 2002, 174(11-2): 111–127. [11] SAXENA S, KAHANDAWALA M S P, SIDHU S S. A shock tube study of ignition delay in the combustion of ethylene [J]. Combustion and Flame, 2011, 158(6): 1019–1031. DOI: 10.1016/j.combustflame.2010.10.011. [12] 梁金虎. 煤油点火延时特性及其污染效应的激波管研究[D]. 重庆: 重庆大学, 2011.LIANG J H. Ignition delay characteristics study of kerosene and contaminated kerosene in shock tube [D]. Chongqing: Chongqing University, 2011. [13] DENG F Q, PAN Y S, SUM W C, et al. Comparative study of the effects of nitrous oxide and oxygen on ethylene ignition [J]. Energy and Fuels, 2017, 31(12): 14116–14128. DOI: 10.1021/acs.energyfuels.7b01425. [14] 廖钦. 煤油及其裂解产物自点火现象的初步实验研究[D]. 合肥: 中国科学技术大学, 2009.LIAO Q. Experimental studies on auto-ignition phenomena of kerosene and cracked kerosene in a shock tube [D]. Hefei: University of Science and Technology of China, 2009. [15] PENYAZKOV O G, SEVROUK K L, TANGIRALA V, et al. High-pressure ethylene oxidation behind reflected shock waves [J]. Proceedings of the Combustion Institute, 2009, 32(2): 2421–2428. DOI: 10.1016/j.proci.2008.06.194. [16] SMITH G P, GOLDEN D M, FRENKLACH M, et al. An optimized detailed chemical reaction mechanism for methane combustion: PB-96-137054/XAB[R]. SRI International, 1995. [17] SARATHY S M, WESTBROOK C K, MEHL M, et al. Comprehensive chemical kinetic modeling of the oxidation of 2-methylalkanes from C7 to C20 [J]. Combustion and Flame, 2011, 158: 2338–2357. DOI: 10.1016/j.combustflame.2011.05.007. [18] JOSHI A, YOU X Q, BARCKHOLTZ T A, et al. Thermal decomposition of ethylene oxide: potential energy surface, master equation analysis, and detailed kinetic modeling [J]. Journal of Physical Chemistry A, 2005, 109(35): 8016–8027. DOI: 10.1021/jp0516442. [19] DAVIDSON D F, HANSON R K. Interpreting shock tube ignition data [J]. International Journal of Chemical Kinetics, 2004, 36(9): 510–523. DOI: 10.1002/kin.20024. [20] WURMEL J, SILKE E J, CURRAN H J, et al. The effect of diluent gases on ignition delay times in the shock tube and in the rapid compression machine [J]. Combustion and Flame, 2007, 151(1-2): 289–302. DOI: 10.1016/j.combustflame.2007.06.010. -






 下载:
下载: