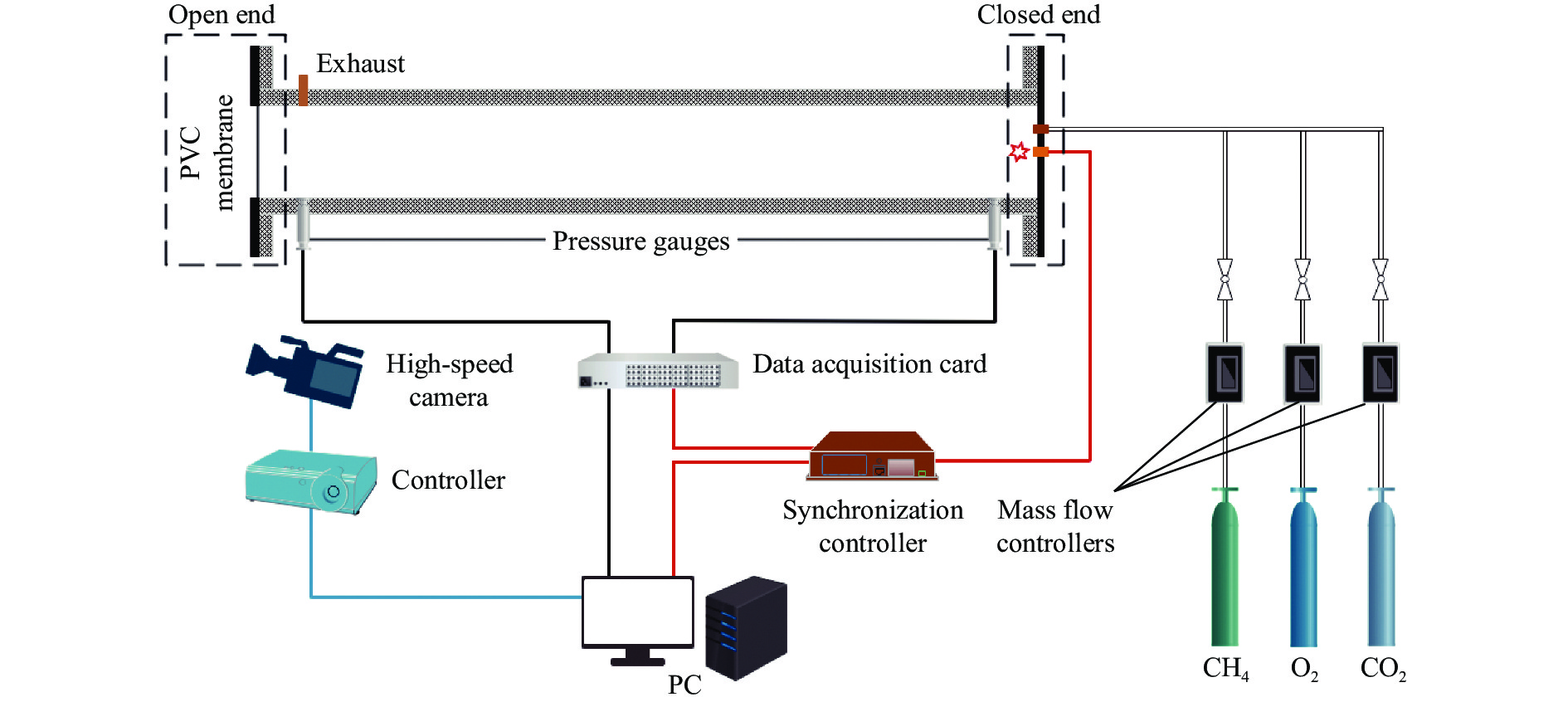
A study of explosion dynamics of a CH4/O2/CO2 premixed system
-
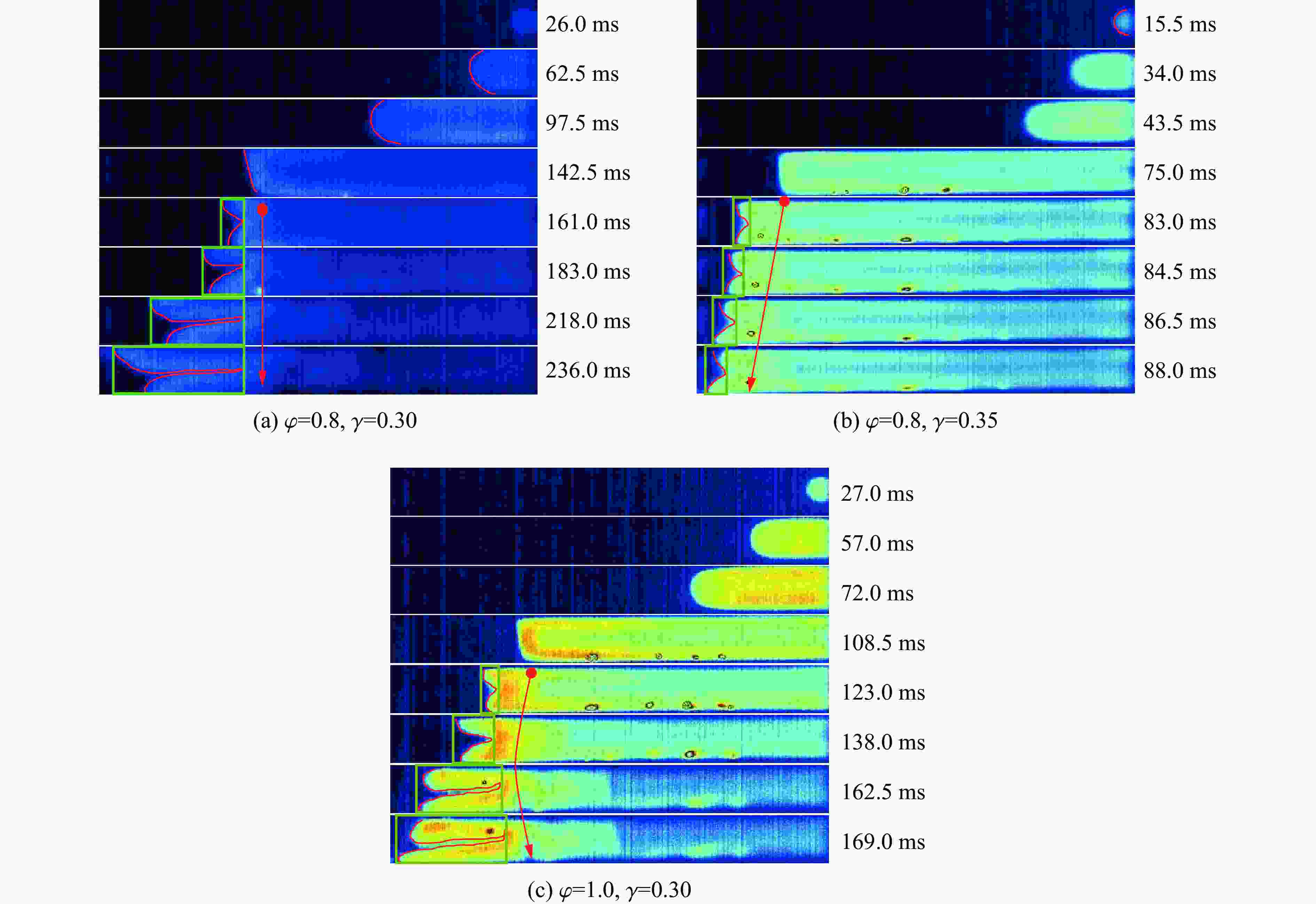
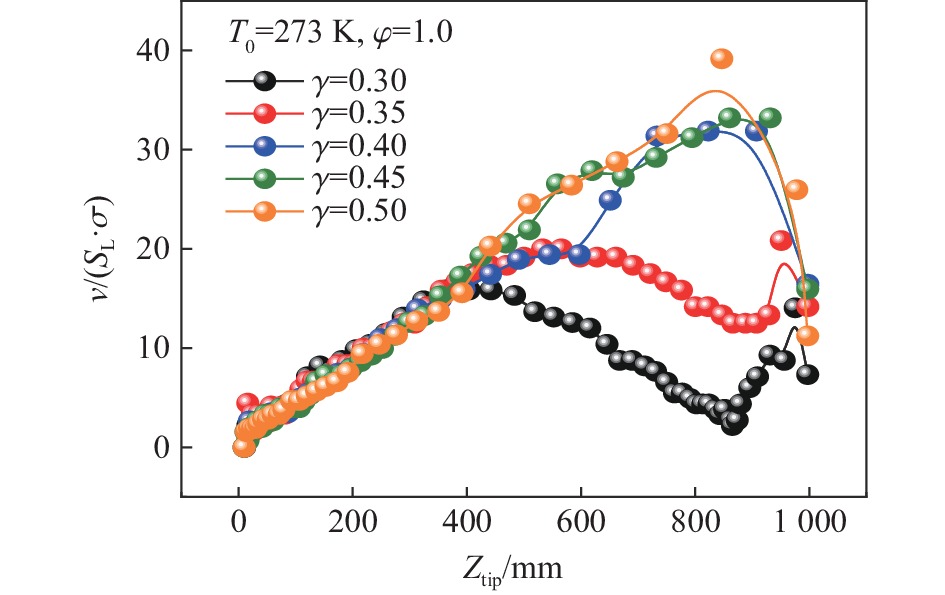
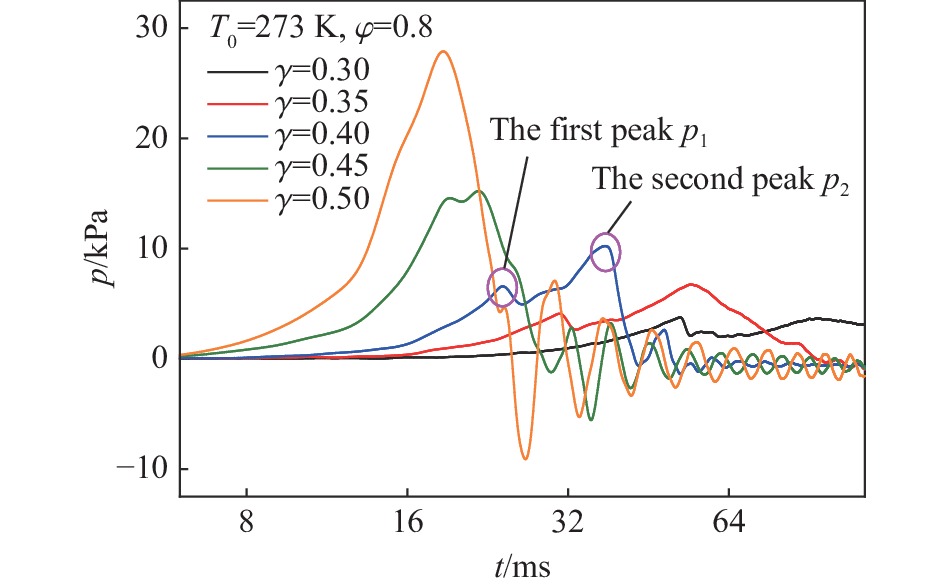
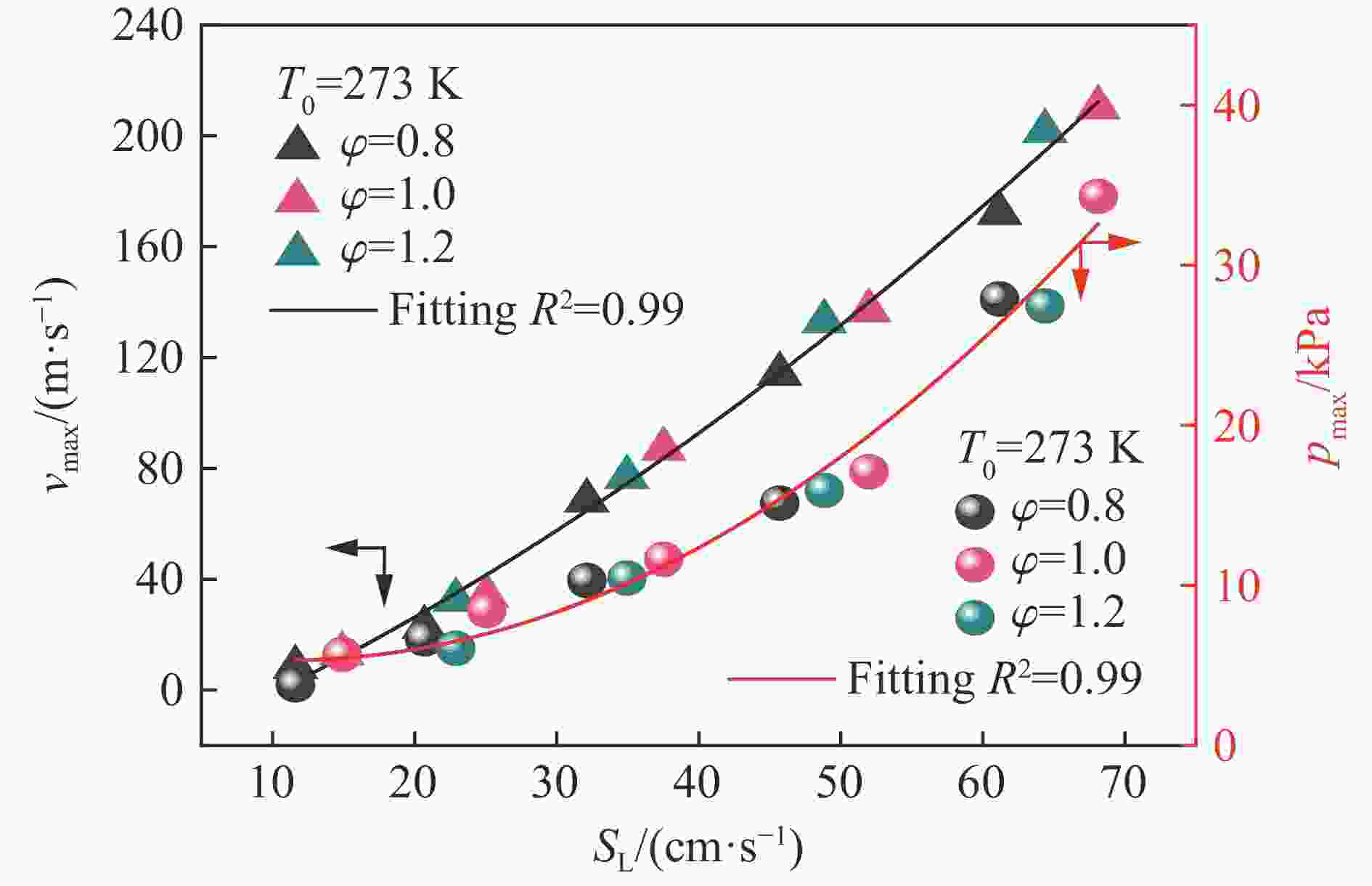
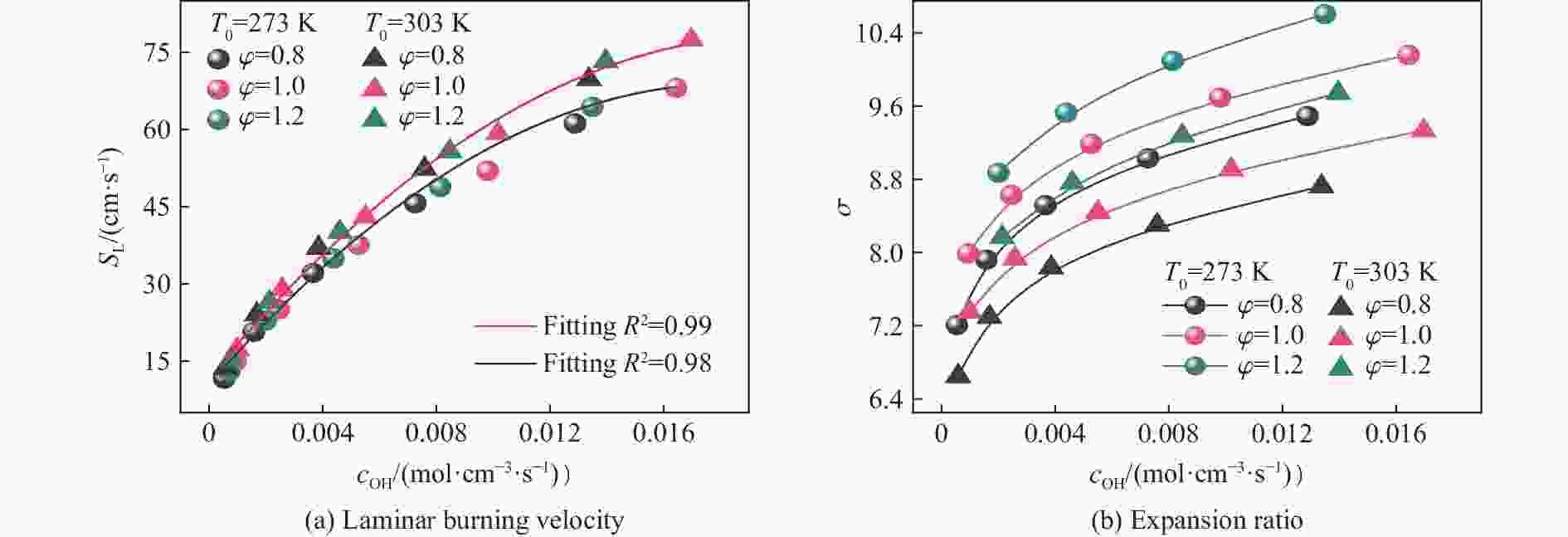
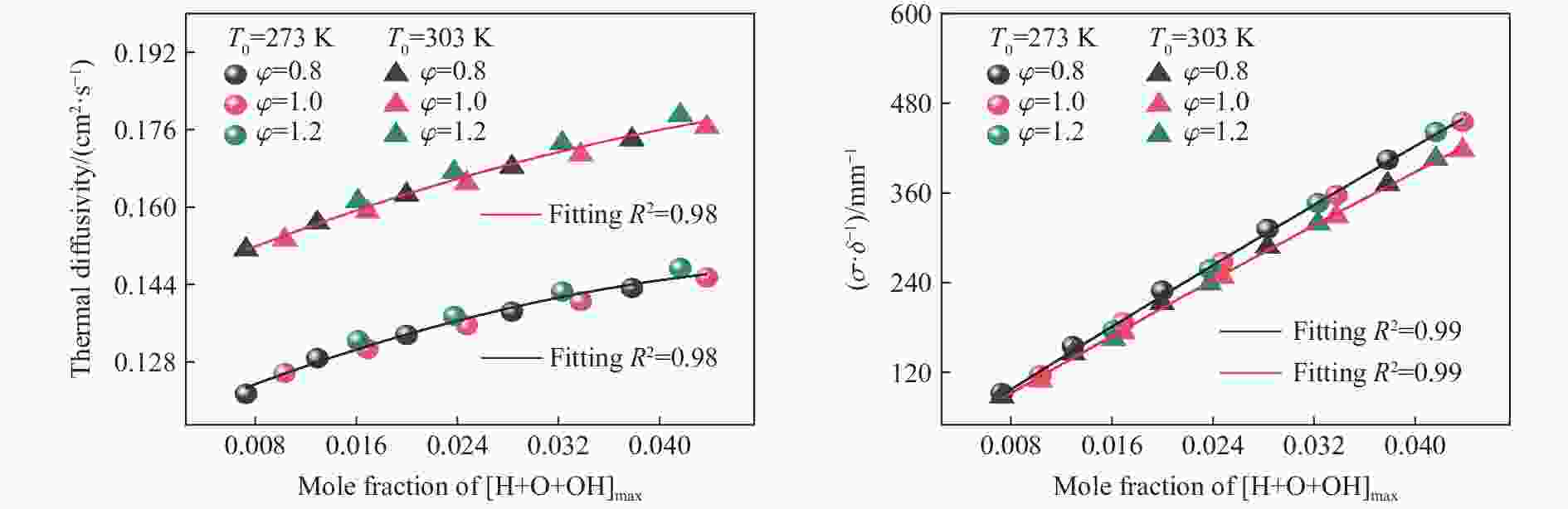
摘要: 为探究甲烷在富氧条件下的火焰动力学规律,以CH4/O2/CO2预混体系为研究对象,在小尺度方形透明管道中进行了一系列爆炸实验,探讨了初始环境温度波动对爆炸参数的影响,并对预混体系的燃烧机理进行分析。结果表明:在273 K的环境温度下,化学当量比
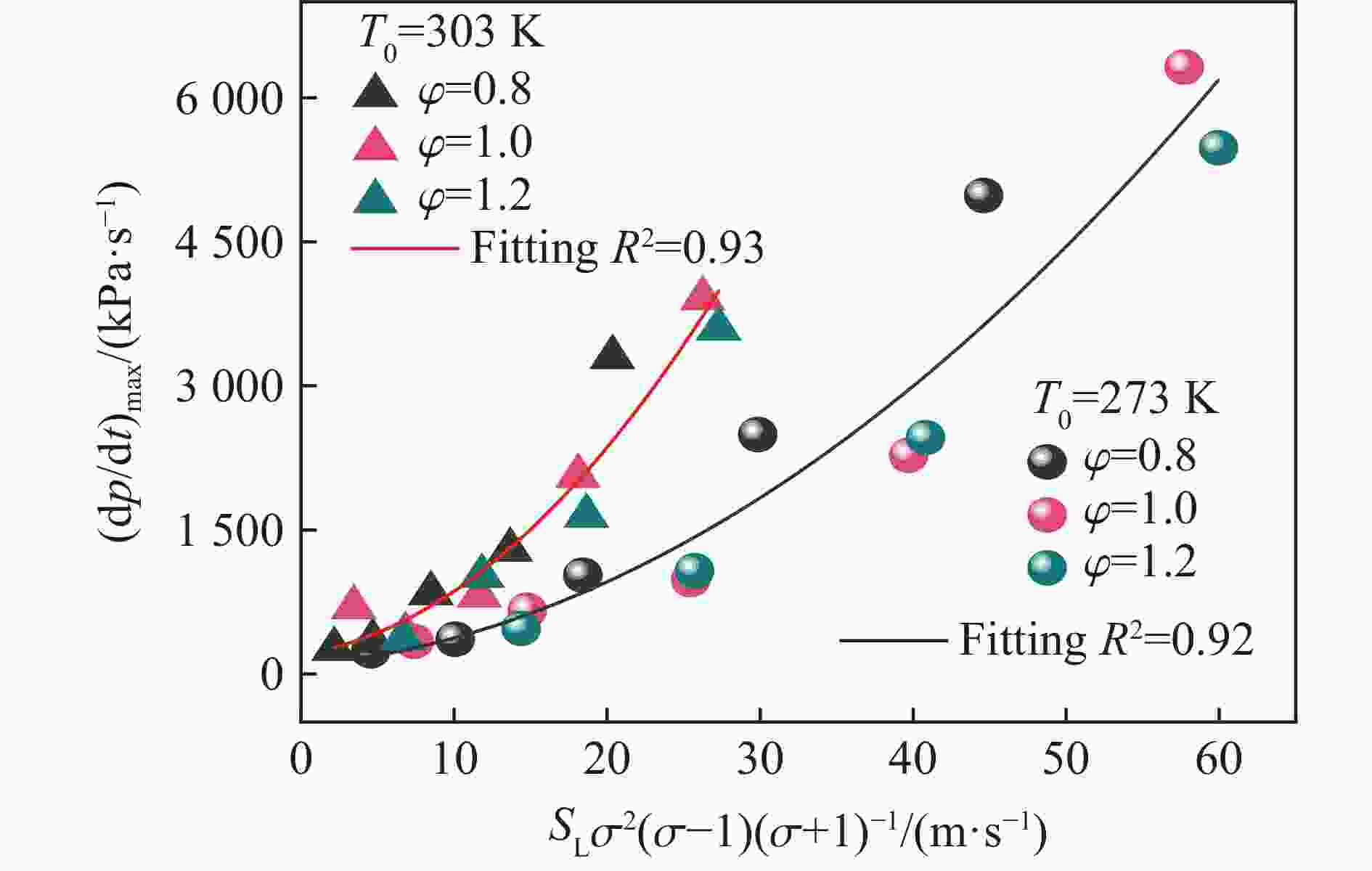
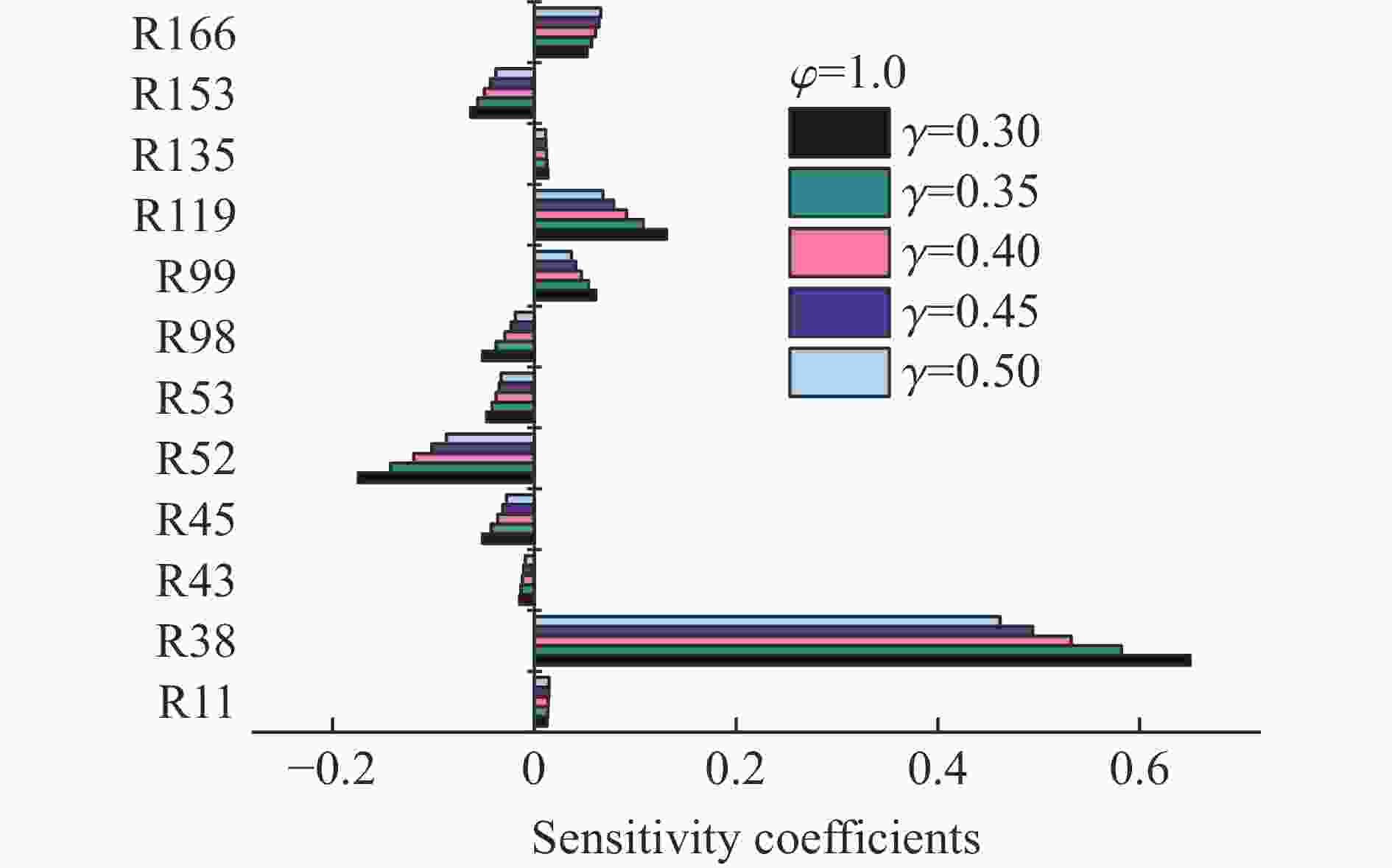
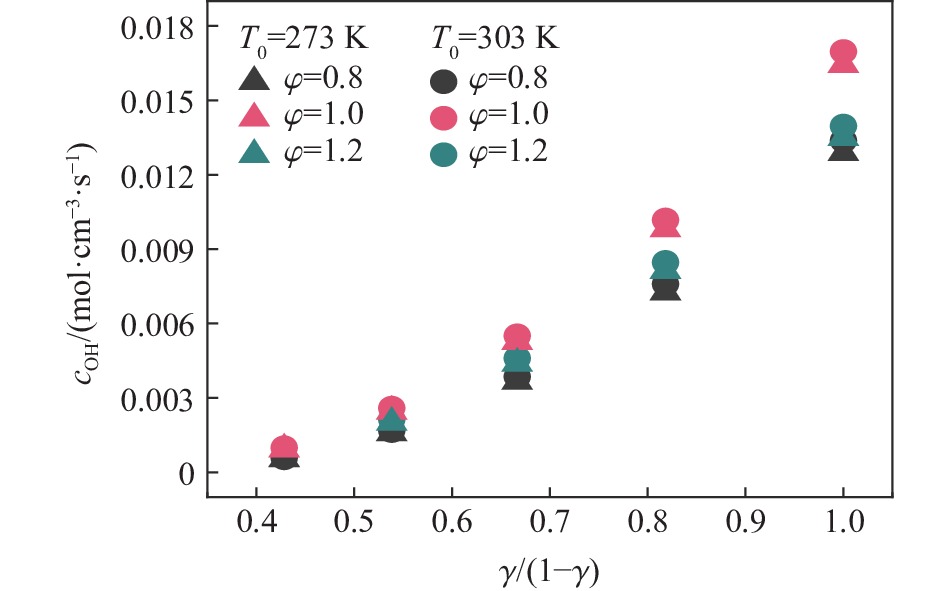
$\varphi $ =0.8~1.0且氧气相对比γ<0.30和$\varphi $ =1.2且γ<0.35的预混体系不能被点燃,而其他预混体系均可被点燃,最终产生郁金香与非郁金香两种火焰类型,并且根据郁金香火焰独特的演变特征,又划分为T形郁金香火焰和不对称郁金香火焰;随着γ的增大,无量纲火焰传播速度v/(SLσ)的变化趋势由“两升两降”转变为“一升一降”。初始环境温度的升高并未对火焰传播速度和爆炸超压的变化趋势产生影响,但是会导致最大爆炸超压pmax和最大火焰传播速度降低。值得注意的是,初始环境温度对爆炸强度的影响随化学当量比的减小而增强。另外,与最大爆炸超压相比,最大火焰传播速度与层流燃烧速度之间的关系更紧密。从敏感性分析中可知:层流燃烧速度对自由基链式反应R38(即H+O2=O+OH)表现出最大的正敏感度,对R52(即H+CH3 (+M)=CH4 (+M))表现出最大的负敏感度,并且对自由基OH的生成速率最敏感,当初始环境温度升高至303 K时,层流燃烧速度对R38(正)和R52(负)的敏感度降低;H、O和OH自由基总摩尔分数的增大会削弱热扩散的不稳定性,增强流体力学的不稳定性。Abstract: In order to explore the flame dynamics of methane under oxygen-rich conditions, a series of explosion experiments were carried out in a small-scale square transparent pipe with a CH4/O2/CO2 premixed system as the research object. Through the analysis of explosion parameters, the influence of the fluctuation of initial ambient temperature on explosion intensity was revealed, and the micro-combustion mechanism of premixed system was discussed. The results show that under the ambient temperature of 273 K, the mixtures with the equivalence ratio$\varphi $ from 0.8 to 1.0, the oxygen relative ratio γ<0.30 and$\varphi $ =1.2, γ<0.35 could not be ignited, but other premixed systems could, and tulip and non-tulip flames were produced. According to the unique evolution characteristics of the tulip flame, the tulip flame can be divided into T-shaped tulip flame and asymmetric tulip flame. As the magnitude of γ increases, the evolution of the maximum normalized flame propagation velocity shifts from a two rises-and-two drops mode to a one rise-and-one drop mode. The increase in the initial ambient temperature has no effect on the evolutions of flame propagation velocity and explosion overpressure, but it reduces the maximum explosion overpressure and the maximum flame propagation velocity. It is worth noting that when the equivalence ratio is lower, the initial ambient temperature has stronger influence on the explosion intensity. In addition, compared with the maximum explosion overpressure, the maximum flame propagation velocity displays a closer relationship with laminar burning velocity. The chemical kinetics calculations show that the laminar burning velocity is most positively sensitive to the free-radical chain reaction R38 (namely, H+O2=O+OH) and is most negatively sensitive to R52 (namely, H+CH3 (+M)=CH4 (+M)), and is most sensitive to the rate of production of the free radical OH. When the initial ambient temperature increases to 303 K, the sensitivity of the laminar burning velocity to R38 (positive) and R52 (negative) are reduced. The increase in the total mole fraction of the free radicals H, O and OH weakens the thermal diffusion instability, but enhances the hydrodynamic instability. -
表 1 临界未点火预混体系甲烷体积分数
Table 1. Critical volume fractions of methane in unignited premixed systems
气体组分 临界未点火预混体系甲烷体积分数/% $\varphi $ =0.8, γ =0.25 $\varphi $ =1.0, γ =0.25 $\varphi $ =1.2, γ =0.30 O2 22.73 22.22 25.43 CH4 9.09 11.11 15.26 表 2
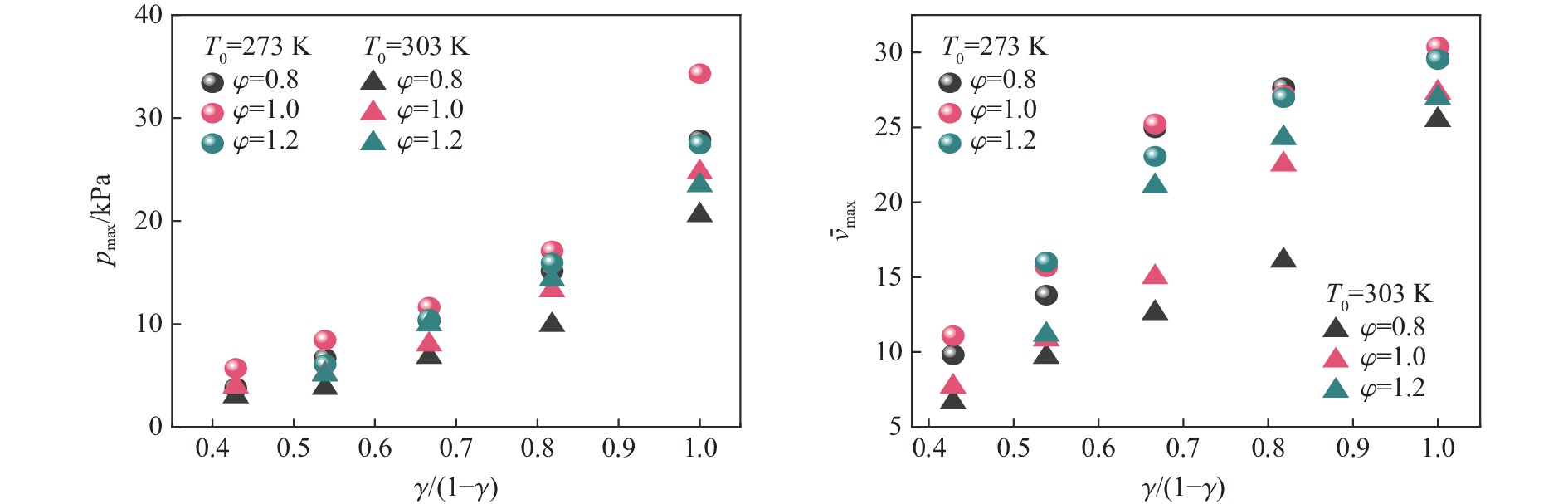
$p_{\max} $ 和$ {\bar v_{{\text{max}}}} $ 在环境温度由$273\;{\rm{K}} $ 升高到$303\;{\rm{K}} $ 影响下的下降百分比Table 2. Decrease percentage of pmax and
$ {\bar v_{{\text{max}}}} $ affected by ambient temperature from$273\;{\rm{K}} $ to$303\;{\rm{K}} $ γ pmax下降百分比/% $ {\bar v_{{\text{max}}}} $下降百分比/% $\varphi $=0.8 $\varphi $=1.0 $\varphi $=1.2 $\varphi $=0.8 $\varphi $=1.0 $\varphi $=1.2 0.30 21.94 31.16 32.33 30.76 0.35 43.38 38.82 16.43 29.81 30.95 30.32 0.40 34.06 31.23 4.77 49.54 40.56 8.65 0.45 34.66 22.45 10.05 41.76 17.08 10.03 0.50 26.30 27.94 14.56 14.02 10.07 8.67 表 3 预混体系中主要的链式反应
Table 3. Main chain reactions in the premixed system
反应序号 主要链式反应 反应序号 主要链式反应 R11 O+CH4=OH+CH3 R98 OH+CH4=CH3+H2O R38 H+O2=O+OH R99 OH+CO=H+CO2 R43 H+OH+M=H2O+M R119 HO2+CH3=OH+CH3O R45 H+H2O=H2+OH R135 CH2+O2=OH+H+CO R52 H+CH3 (+M)=CH4 (+M) R153 CH2(s)+CO2=CH2O+CO R53 H+CH4=CH3+H2 R166 HCO+H2O=H+CO+H2O -
[1] SAANUM I, DITARANTO M. Experimental study of oxy-fuel combustion under gas turbine conditions [J]. Energy and Fuels, 2017, 31(4): 4445–4451. DOI: 10.1021/acs.energyfuels.6b03114. [2] STANGER R, WALL T, SPÖRL R, et al. Oxyfuel combustion for CO2 capture in power plants [J]. International Journal of Greenhouse Gas Control, 2015, 40: 55–125. DOI: 10.1016/j.ijggc.2015.06.010. [3] CHAKROUN N W, GHONIEM A F. High-efficiency low LCOE combined cycles for sour gas oxy-combustion with CO2 capture [J]. International Journal of Greenhouse Gas Control, 2015, 41: 163–173. DOI: 10.1016/j.ijggc.2015.06.025. [4] LI B, SHI B, CHU Q, et al. Characteristics of stoichiometric CH4/O2/CO2 flame up to the pure oxygen condition [J]. Energy, 2019, 168: 151–159. DOI: 10.1016/j.energy.2018.11.039. [5] ABDELHAFEZ A, NEMITALLAH M A, RASHWAN S S, et al. Adiabatic flame temperature for controlling the macrostructures and stabilization modes of premixed methane flames in a model gas-turbine combustor [J]. Energy and Fuels, 2018, 32(7): 7868–7877. DOI: 10.1021/acs.energyfuels.8b01133. [6] KUTNE P, KAPADIA B K, MEIER W, et al. Experimental analysis of the combustion behaviour of oxyfuel flames in a gas turbine model combustor [J]. Proceedings of the Combustion Institute, 2011, 33(2): 3383–3390. DOI: 10.1016/j.proci.2010.07.008. [7] LIU C Y, CHEN G, SIPÖCZ N, et al. Characteristics of oxy-fuel combustion in gas turbines [J]. Applied Energy, 2012, 89(1): 387–394. DOI: 10.1016/j.apenergy.2011.08.004. [8] ZHANG Q S, CHEN G Y, DENG H X, et al. Experimental and numerical study of the effects of oxygen-enriched air on the laminar burning characteristics of biomass-derived syngas [J]. Fuel, 2021, 285: 119183. DOI: 10.1016/j.fuel.2020.119183. [9] HU X Z, YU Q B, SUN N, et al. Effects of high concentrations of CO2 on the lower flammability limits of oxy-methane mixtures [J]. Energy and Fuels, 2016, 30(5): 4346–4352. DOI: 10.1021/acs.energyfuels.6b00492. [10] HU X Z, YU Q B, LIU J X, et al. Investigation of laminar flame speeds of CH4/O2/CO2 mixtures at ordinary pressure and kinetic simulation [J]. Energy, 2014, 70: 626–634. DOI: 10.1016/j.energy.2014.04.029. [11] DI BENEDETTO A, CAMMAROTA F, DI SARLI V, et al. Anomalous behavior during explosions of CH4 in oxygen-enriched air [J]. Combustion and Flame, 2011, 158(11): 2214–2219. DOI: 10.1016/j.combustflame.2011.03.015. [12] DI BENEDETTO A, DI SARLI V, SALZANO E, et al. Explosion behavior of CH4/O2/N2/CO2 and H2/O2/N2/CO2 mixtures [J]. International Journal of Hydrogen Energy, 2009, 34(16): 6970–6978. DOI: 10.1016/j.ijhydene.2009.05.120. [13] XIA Y, HASHIMOTO G, HADI K, et al. Turbulent burning velocity of ammonia/oxygen/nitrogen premixed flame in O2-enriched air condition [J]. Fuel, 2020, 268: 117383. DOI: 10.1016/j.fuel.2020.117383. [14] CAI X, WANG J H, ZHANG W J, et al. Effects of oxygen enrichment on laminar burning velocities and Markstein lengths of CH4/O2/N2 flames at elevated pressures [J]. Fuel, 2016, 184: 466–473. DOI: 10.1016/j.fuel.2016.07.011. [15] CHAN Y L, ZHU M M, ZHANG Z Z, et al. The effect of CO2 dilution on the laminar burning velocity of premixed methane/air flames [J]. Energy Procedia, 2015, 75: 3048–3053. DOI: 10.1016/j.egypro.2015.07.621. [16] KHAN A R, ANBUSARAVANAN S, KALATHI L, et al. Investigation of dilution effect with N2/CO2 on laminar burning velocity of premixed methane/oxygen mixtures using freely expanding spherical flames [J]. Fuel, 2017, 196: 225–232. DOI: 10.1016/j.fuel.2017.01.086. [17] LIU F, GUO H S, SMALLWOOD G J. The chemical effect of CO2 replacement of N2 in air on the burning velocity of CH4 and H2 premixed flames [J]. Combustion and Flame, 2003, 133(4): 495–497. DOI: 10.1016/S0010-2180(03)00019-1. [18] ZHANG C, SHEN X B, WEN J X, et al. The behavior of methane/hydrogen/air premixed flame in a closed channel with inhibition [J]. Fuel, 2020, 265: 116810. DOI: 10.1016/j.fuel.2019.116810. [19] ZHANG C, WEN J, SHEN X B, et al. Experimental study of hydrogen/air premixed flame propagation in a closed channel with inhibitions for safety consideration [J]. International Journal of Hydrogen Energy, 2019, 44(40): 22654–22660. DOI: 10.1016/j.ijhydene.2019.04.032. [20] HU X Z, YU Q B, LIU J X. Chemical effect of CO2 on the laminar flame speeds of oxy-methane mixtures in the condition of various equivalence ratios and oxygen concentrations [J]. International Journal of Hydrogen Energy, 2016, 41(33): 15068–15077. DOI: 10.1016/j.ijhydene.2016.05.276. [21] BAI C H, LIU W J, YAO J, et al. Explosion characteristics of liquid fuels at low initial ambient pressures and temperatures [J]. Fuel, 2020, 265: 116951. DOI: 10.1016/j.fuel.2019.116951. [22] GRABARCZYK M, TEODORCZYK A, DI SARLI V, et al. Effect of initial temperature on the explosion pressure of various liquid fuels and their blends [J]. Journal of Loss Prevention in the Process Industries, 2016, 44: 775–779. DOI: 10.1016/j.jlp.2016.08.013. [23] NIE B S, YANG L L, GE B Q, et al. Chemical kinetic characteristics of methane/air mixture explosion and its affecting factors [J]. Journal of Loss Prevention in the Process Industries, 2017, 49: 675–682. DOI: 10.1016/j.jlp.2017.02.021. [24] CHU H Q, XIANG L K, MENG S, et al. Effects of N2 dilution on laminar burning velocity, combustion characteristics and NO x emissions of rich CH4-air premixed flames [J]. Fuel, 2021, 284: 119017. DOI: 10.1016/j.fuel.2020.119017. [25] LI J, HUANG H Y, KOBAYASHI N, et al. Numerical study on effect of oxygen content in combustion air on ammonia combustion [J]. Energy, 2015, 93: 2053–2068. DOI: 10.1016/j.energy.2015.10.060. [26] ZHENG L G, DU D P, WANG J, et al. Study on premixed flame dynamics of CH4/O2/CO2 mixtures [J]. Fuel, 2019, 256: 115913. DOI: 10.1016/j.fuel.2019.115913. [27] 李成兵. N2/CO2/H2O抑制甲烷爆炸化学动力学机理分析 [J]. 中国安全科学学报, 2010, 20(8): 88–92. DOI: 10.3969/j.issn.1003-3033.2010.08.014.LI C B. Chemical kinetics mechanism analysis of N2/CO2/H2O suppressing methane explosion [J]. China Safety Science Journal, 2010, 20(8): 88–92. DOI: 10.3969/j.issn.1003-3033.2010.08.014. [28] 高娜, 张延松, 胡毅亭. 温度、压力对甲烷-空气混合物爆炸极限耦合影响的实验研究 [J]. 爆炸与冲击, 2017, 37(3): 453–458. DOI: 10.11883/1001-1455(2017)03-0453-06.GAO N, ZHANG Y S, HU Y T. Experimental study on methane-air mixtures explosion limits at normal and elevated initial temperatures and pressures [J]. Explosion and Shock Waves, 2017, 37(3): 453–458. DOI: 10.11883/1001-1455(2017)03-0453-06. [29] CLANET C, SEARBY G. On the tulip flame phenomenon [J]. Combustion and Flame, 1996, 105(1/2): 225–238. DOI: 10.1016/0010-2180(95)00195-6. [30] SHEN X B, ZHANG C, XIU G, et al. Evolution of premixed stoichiometric hydrogen/air flame in a closed duct [J]. Energy, 2019, 176: 265–271. DOI: 10.1016/j.energy.2019.03.193. [31] XIAO H H, SHEN X B, SUN J H. Experimental study and three-dimensional simulation of premixed hydrogen/air flame propagation in a closed duct [J]. International Journal of Hydrogen Energy, 2012, 37(15): 11466–11473. DOI: 10.1016/j.ijhydene.2012.05.006. [32] XIAO H H, HOUIM R W, ORAN E S. Formation and evolution of distorted tulip flames [J]. Combustion and Flame, 2015, 162(11): 4084–4101. DOI: 10.1016/j.combustflame.2015.08.020. [33] PONIZY B, CLAVERIE A, VEYSSIÈRE B. Tulip flame: the mechanism of flame front inversion [J]. Combustion and Flame, 2014, 161(12): 3051–3062. DOI: 10.1016/j.combustflame.2014.06.001. [34] GONZALEZ M, BORGHI R, SAOUAB A. Interaction of a flame front with its self-generated flow in an enclosure: the tulip flame phenomenon [J]. Combustion and Flame, 1992, 88(2): 201–220. DOI: 10.1016/0010-2180(92)90052-Q. [35] DUNN-RANKIN D, SAWYER R F. Tulip flames: changes in shape of premixed flames propagating in closed tubes [J]. Experiments in Fluids, 1998, 24(2): 130–140. DOI: 10.1007/s003480050160. [36] 李艳超, 毕明树, 高伟. 耦合火焰不稳定的爆炸超压预测 [J]. 爆炸与冲击, 2020, 40(1): 012101. DOI: 10.11883/bzycj-2019-0004.LI Y C, BI M S, GAO W. Explosion pressure prediction considering the flame instabilities [J]. Explosion and Shock Waves, 2020, 40(1): 012101. DOI: 10.11883/bzycj-2019-0004. [37] 吕启申, 臧小为, 潘旭海, 等. 温度和浓度对甲醇喷雾爆炸特性参数的影响 [J]. 爆炸与冲击, 2019, 39(9): 095402. DOI: 10.11883/bzycj-2018-0121.LYU Q S, ZANG X W, PAN X H, et al. Effects of temperature and concentration on characteristic parameters of methanol explosion [J]. Explosion and Shock Waves, 2019, 39(9): 095402. DOI: 10.11883/bzycj-2018-0121. [38] HERNANDEZ F, ABDEL-JAWAD M, HAO H. Simplified multiple equations’ inverse problem of vented vessels subjected to internal gas explosions [J]. Journal of Loss Prevention in the Process Industries, 2015, 35: 65–79. DOI: 10.1016/j.jlp.2015.03.007. [39] BYCHKOV V, AKKERMAN V, FRU G, et al. Flame acceleration in the early stages of burning in tubes [J]. Combustion and Flame, 2007, 150(4): 263–276. DOI: 10.1016/j.combustflame.2007.01.004. [40] WEI S M, YU M G, PEI B, et al. Suppression of CO2 and H2O on the cellular instability of premixed methane/air flame [J]. Fuel, 2020, 264: 116862. DOI: 10.1016/j.fuel.2019.116862. [41] XIANG L K, CHU H Q, REN F, et al. Numerical analysis of the effect of CO2 on combustion characteristics of laminar premixed methane/air flames [J]. Journal of the Energy Institute, 2019, 92(5): 1487–1501. DOI: 10.1016/j.joei.2018.06.018. -








 下载:
下载: