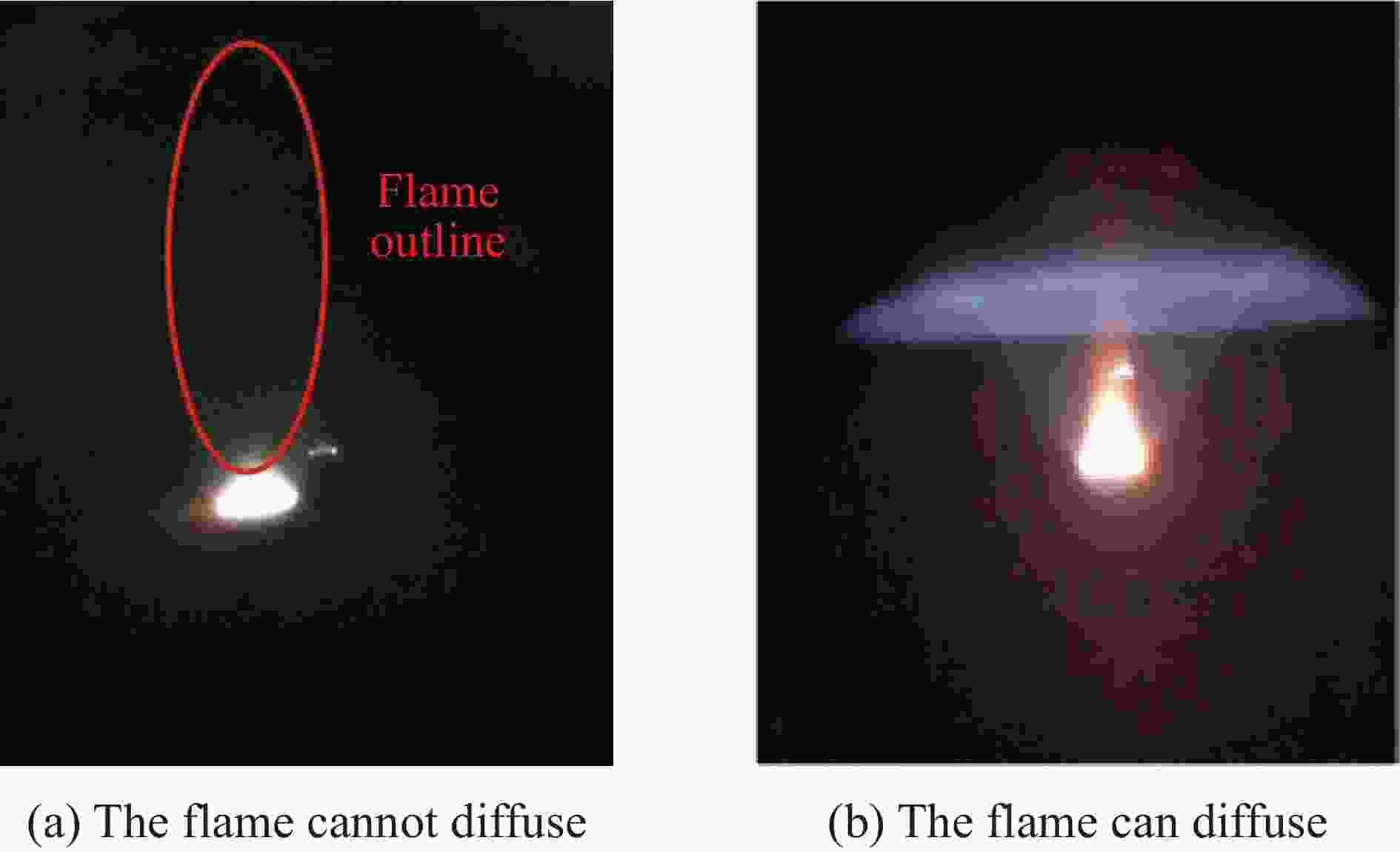
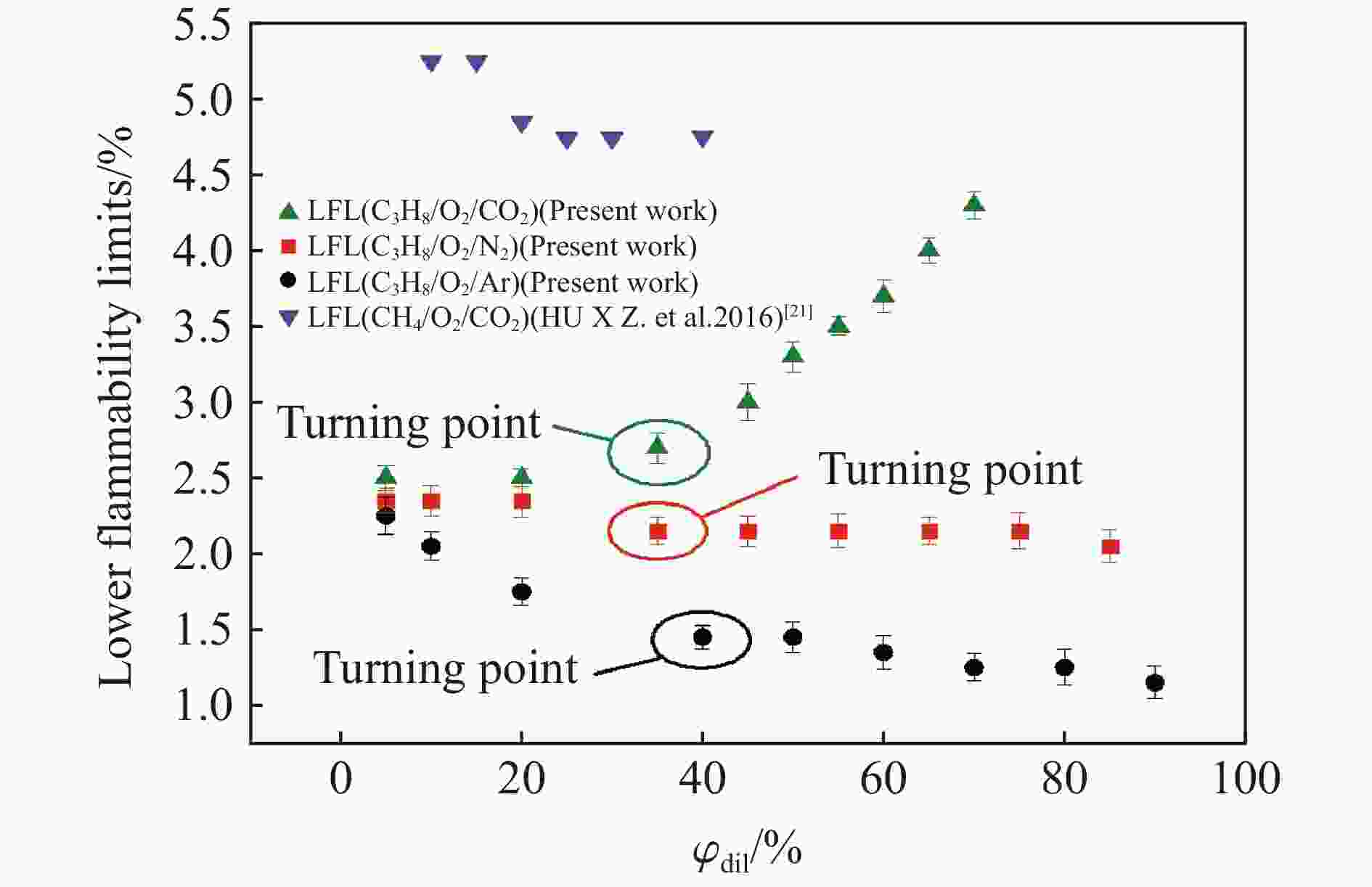
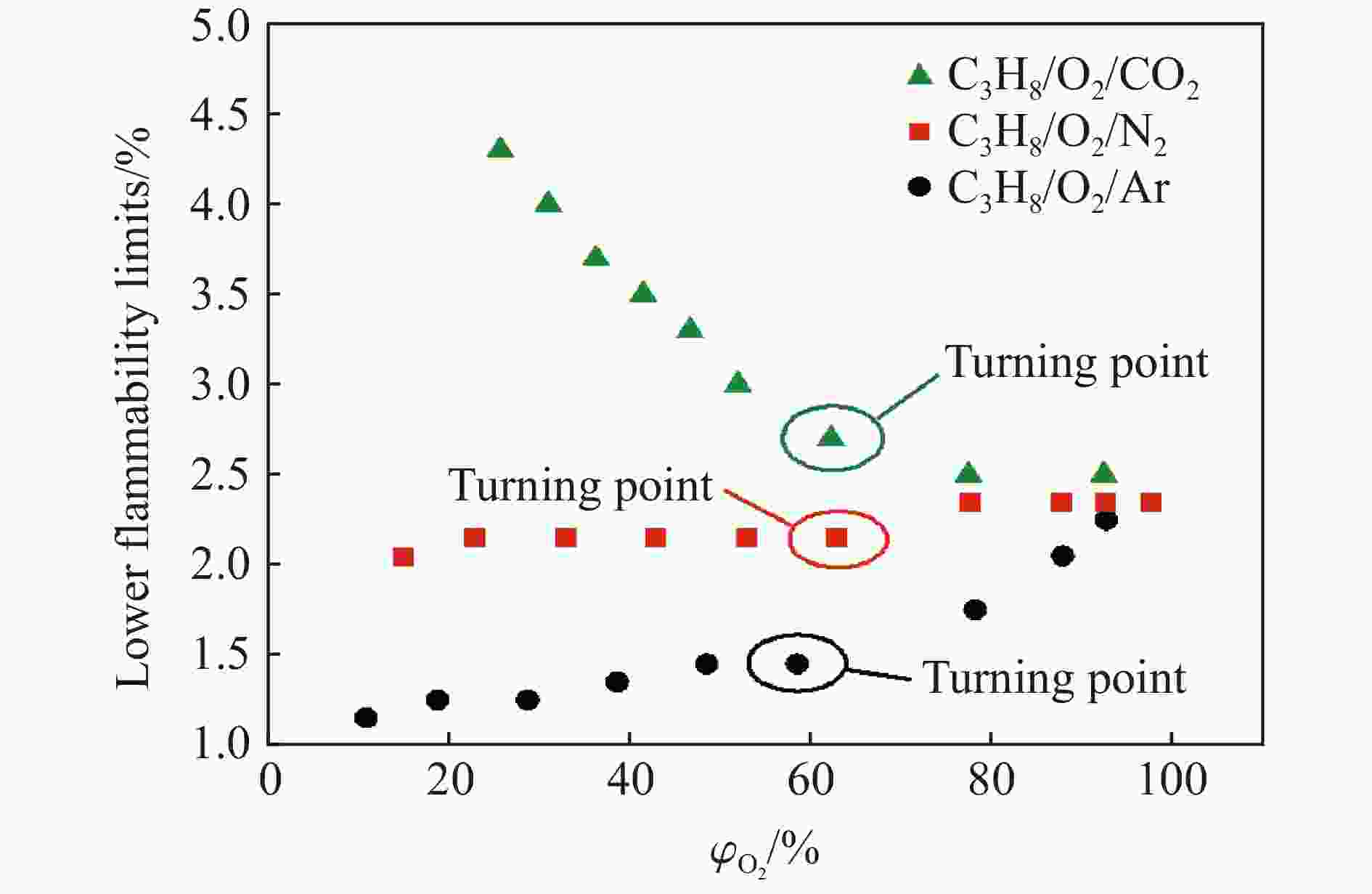
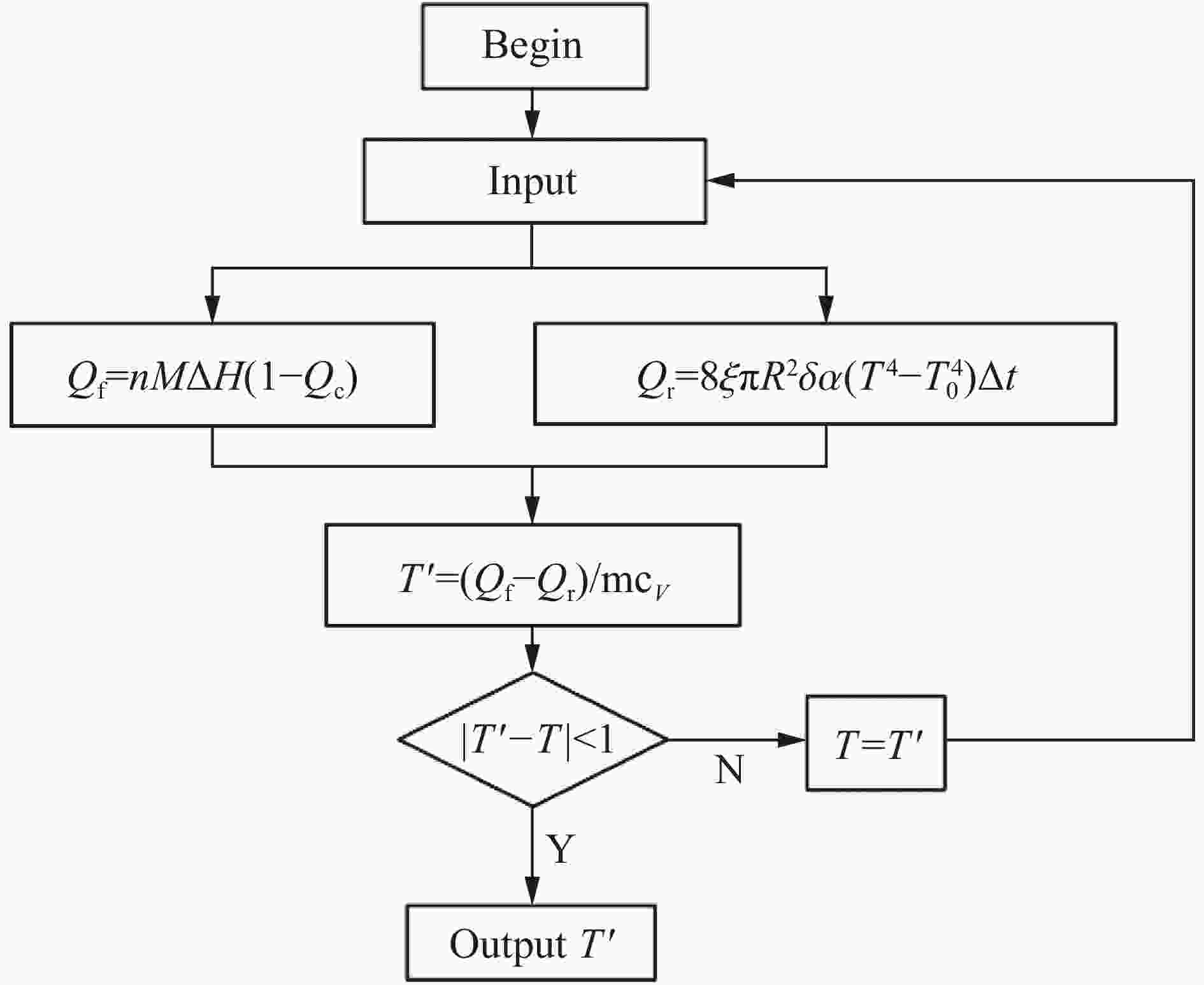
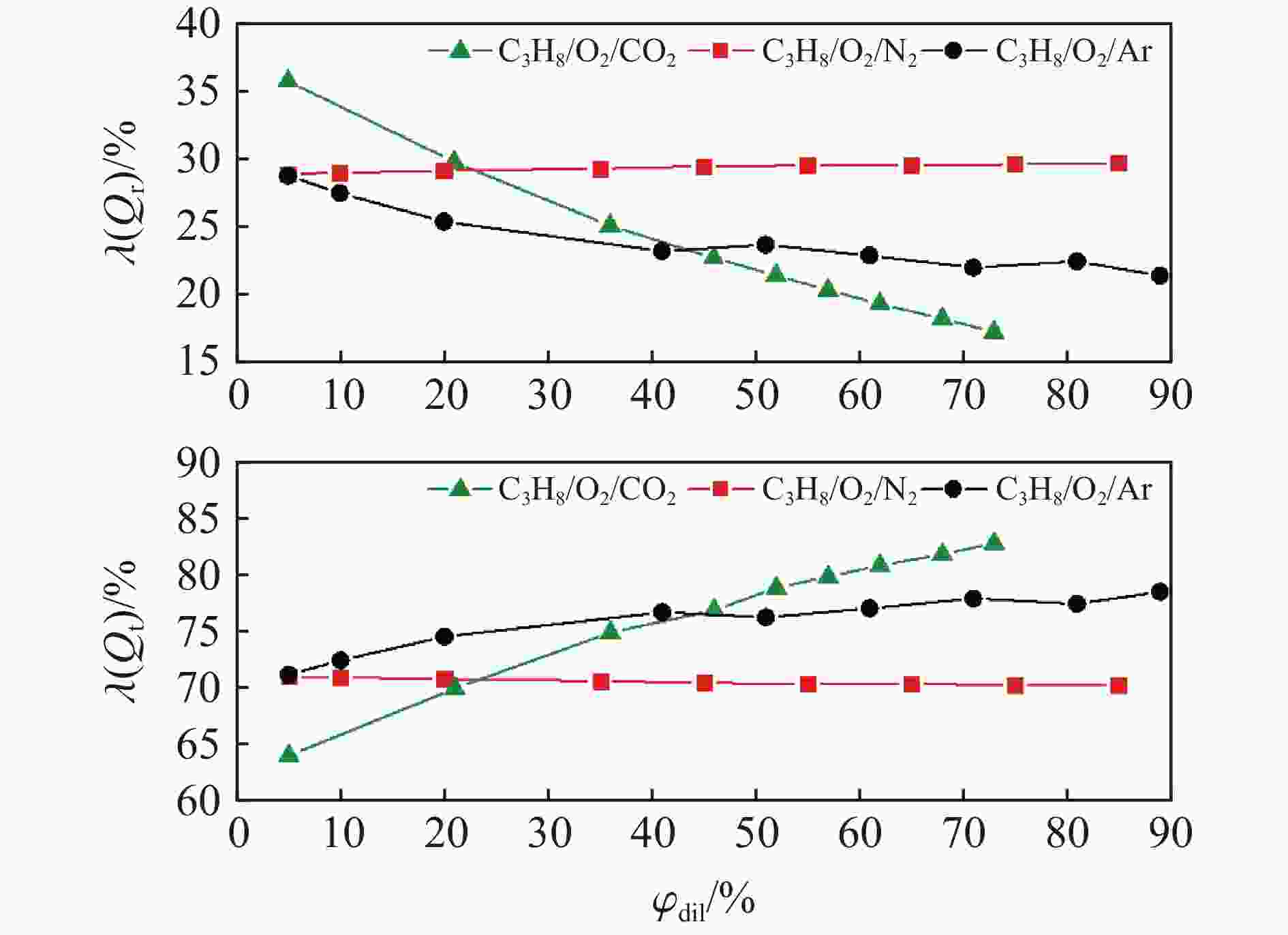
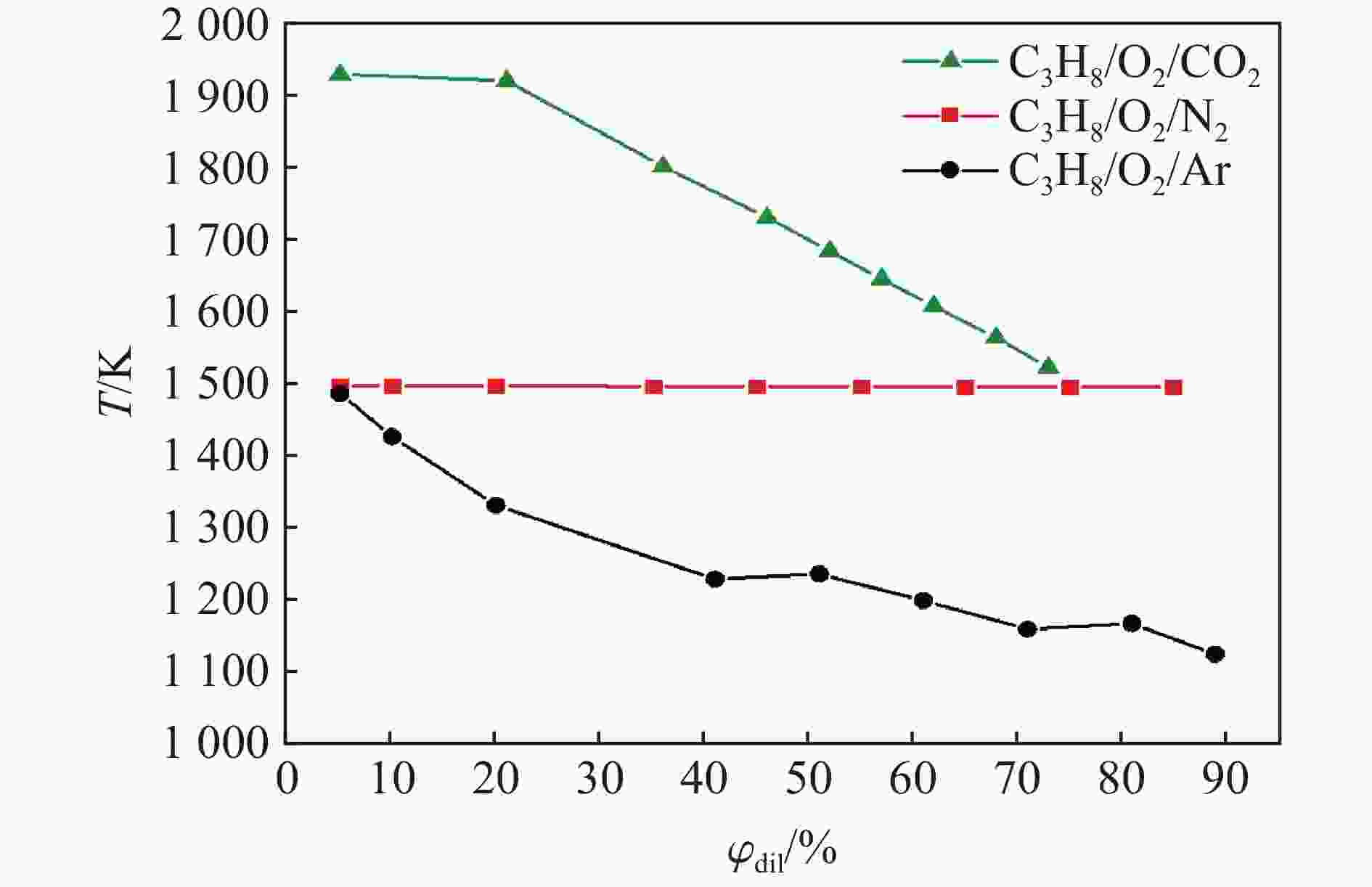
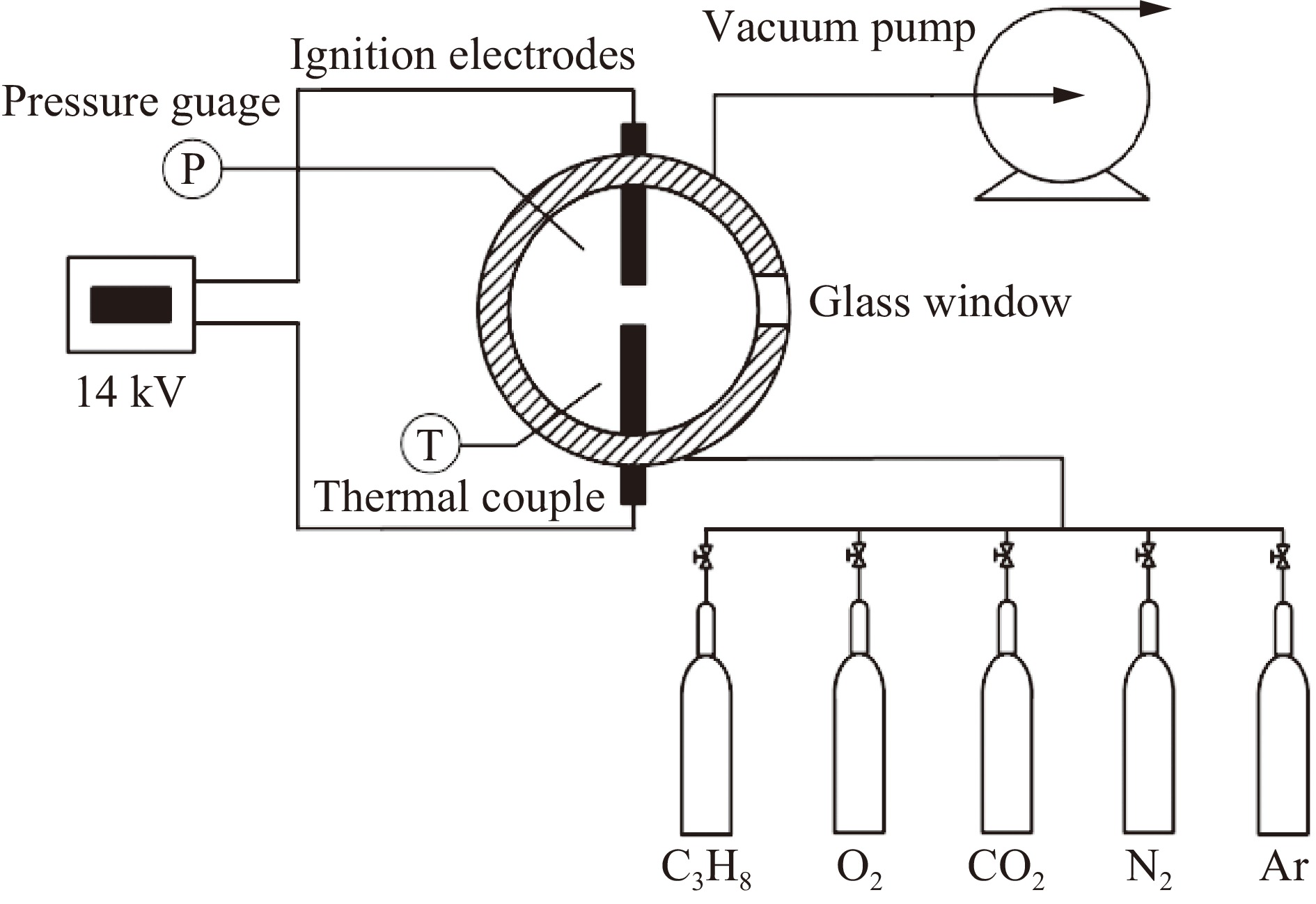
| [1] |
ZHAO Y, LI H N, ZHANG S C, et al. Seismic analysis of a large LNG tank considering different site conditions [J]. Applied Sciences, 2020, 10(22): 8121. DOI: 10.3390/app10228121.
|
| [2] |
GOULD C F, URPELAINEN J. LPG as a clean cooking fuel: adoption, use, and impact in rural India [J]. Energy Policy, 2018, 122: 395–408. DOI: 10.1016/j.enpol.2018.07.042.
|
| [3] |
LOVACHEV L A. The theory of limits on flame propagation in gases [J]. Combustion and Flame, 1971, 17(3): 275–278. DOI: 10.1016/S0010-2180(71)80048-2.
|
| [4] |
FLACK J, BENNETT A J S, STRONG R, et al. Combution theory [M]. Menlo Park: Marine Combustion Practice, 1969: 126−140.
|
| [5] |
WAN X, ZHANG Q, SHEN S L. Theoretical estimation of the lower flammability limit of fuel-air mixtures at elevated temperatures and pressures [J]. Journal of Loss Prevention in the Process Industries, 2015, 36: 13–19. DOI: 10.1016/j.jlp.2015.05.001.
|
| [6] |
FENG B, YANG Z, LV Z J, et al. Effect of gas disturbance on combustion characteristics of flammable refrigerants near LFLs [J]. Journal of Hazardous Materials, 2019, 368: 21–32. DOI: 10.1016/j.jhazmat.2018.12.119.
|
| [7] |
KONDO S, TAKIZAWA K, TAKAHASHI A, et al. On the temperature dependence of flammability limits of gases [J]. Journal of Hazardous Materials, 2011, 187(1/2/3): 585–590. DOI: 10.1016/j.jhazmat.2011.01.037.
|
| [8] |
KIM N I, KATAOKA T, MARUYAMA S, et al. Flammability limits of stationary flames in tubes at low pressure [J]. Combustion and Flame, 2005, 141(1/2): 78–88. DOI: 10.1016/j.combustflame.2004.12.011.
|
| [9] |
CHEN J R, TSAI H Y, CHIEN J H, et al. Flow and flame visualization near the upper flammability limits of methane/air and propane/air mixtures at elevated pressures [J]. Journal of Loss Prevention in the Process Industries, 2011, 24(5): 662–670. DOI: 10.1016/j.jlp.2011.05.012.
|
| [10] |
LUO Z M, SU B, WANG T, et al. Effects of propane on the flammability limits and chemical kinetics of methane–air explosions [J]. Combustion Science and Technology, 2020, 192(9): 1785–1801. DOI: 10.1080/00102202.2019.1625041.
|
| [11] |
MENDIBURU A Z, DE CARVALHO J A JR, CORONADO C R. Method for determination of flammability limits of gaseous compounds diluted with N2 and CO2 in air [J]. Fuel, 2018, 226: 65–80. DOI: 10.1016/j.fuel.2018.03.181.
|
| [12] |
BLINT R J. Flammability limits for exhaust gas diluted flames [J]. Symposium (International) on Combustion, 1989, 22(1): 1547–1554. DOI: 10.1016/S0082-0784(89)80165-1.
|
| [13] |
BUHRE B J P, ELLIOTT L K, SHENG C D, et al. Oxy-fuel combustion technology for coal-fired power generation [J]. Progress in Energy and Combustion Science, 2005, 31(4): 283–307. DOI: 10.1016/j.pecs.2005.07.001.
|
| [14] |
FEDYAEVA O N, ARTAMONOV D O, VOSTRIKOV A A. Effect of H2O and CO2 on propane, propene, and isopropanol oxidation at elevated pressures [J]. Combustion and Flame, 2019, 199: 230–240. DOI: 10.1016/j.combustflame.2018.09.031.
|
| [15] |
GIURCAN V, MITU M, MOVILEANU C, et al. Influence of inert additives on small-scale closed vessel explosions of propane-air mixtures [J]. Fire Safety Journal, 2020, 111: 102939. DOI: 10.1016/j.firesaf.2019.102939.
|
| [16] |
PEKALSKI A A, ZEVENBERGEN J F, LEMKOWITZ S M, et al. A review of explosion prevention and protection systems suitable as ultimate layer of protection in chemical process installations [J]. Process Safety and Environmental Protection, 2005, 83(1): 1–17. DOI: 10.1205/psep.04023.
|
| [17] |
LI Y C, BI M S, HUANG L, et al. Hydrogen cloud explosion evaluation under inert gas atmosphere [J]. Fuel Processing Technology, 2018, 180: 96–104. DOI: 10.1016/j.fuproc.2018.08.015.
|
| [18] |
HU X Z, XIE Q H, YU Q B, et al. Effect of carbon dioxide on the lower flammability limit of propane in O2/CO2 atmosphere [J]. Energy & Fuels, 2020, 34(4): 4993–4998. DOI: 10.1021/acs.energyfuels.0c00601.
|
| [19] |
HU X Z, XIE Q H, ZHANG J, et al. Experimental study of the lower flammability limits of H2/O2/CO2 mixture [J]. International Journal of Hydrogen Energy, 2020, 45(51): 27837–27845. DOI: 10.1016/j.ijhydene.2020.07.033.
|
| [20] |
PIO G, SALZANO E. The effect of ultra-low temperature on the flammability limits of a methane/air/diluent mixtures [J]. Journal of Hazardous Materials, 2019, 362: 224–229. DOI: 10.1016/j.jhazmat.2018.09.018.
|
| [21] |
HU X Z, YU Q B, SUN N, et al. Effects of high concentrations of CO2 on the lower flammability limits of oxy-methane mixtures [J]. Energy & Fuels, 2016, 30(5): 4346–4352. DOI: 10.1021/acs.energyfuels.6b00492.
|
| [22] |
HU S T, WANG P Y, PITZ R W, et al. Experimental and numerical investigation of non-premixed tubular flames [J]. Proceedings of the Combustion Institute, 2007, 31(1): 1093–1099. DOI: 10.1016/j.proci.2006.08.058.
|
| [23] |
YAWS C L. Matheson气体数据手册 [M]. 7版. 陶鹏万, 黄建彬, 朱大方, 译. 北京: 化学工业出版社, 2001: 27−230.
|
| [24] |
张家荣, 赵廷元. 工程常用物质的热物理性质手册 [M]. 北京: 新时代出版社, 1987: 256-301.
|
| [25] |
KANGWANPONGPAN T, FRANCA F H R, DA SILVA R C, et al. New correlations for the weighted-sum-of-gray-gases model in oxy-fuel conditions based on HITEMP 2010 database [J]. International Journal of Heat and Mass Transfer, 2012, 55(25/26): 7419–7433. DOI: 10.1016/j.ijheatmasstransfer.2012.07.032.
|
| [26] |
HU X Z, YU Q B, SUN Y S. Effects of carbon dioxide on the upper flammability limits of methane in O2/CO2 atmosphere [J]. Energy, 2020, 208: 118417. DOI: 10.1016/j.energy.2020.118417.
|






 下载:
下载: