Effect of external magnetic field on explosion reaction of acetylene gas
-
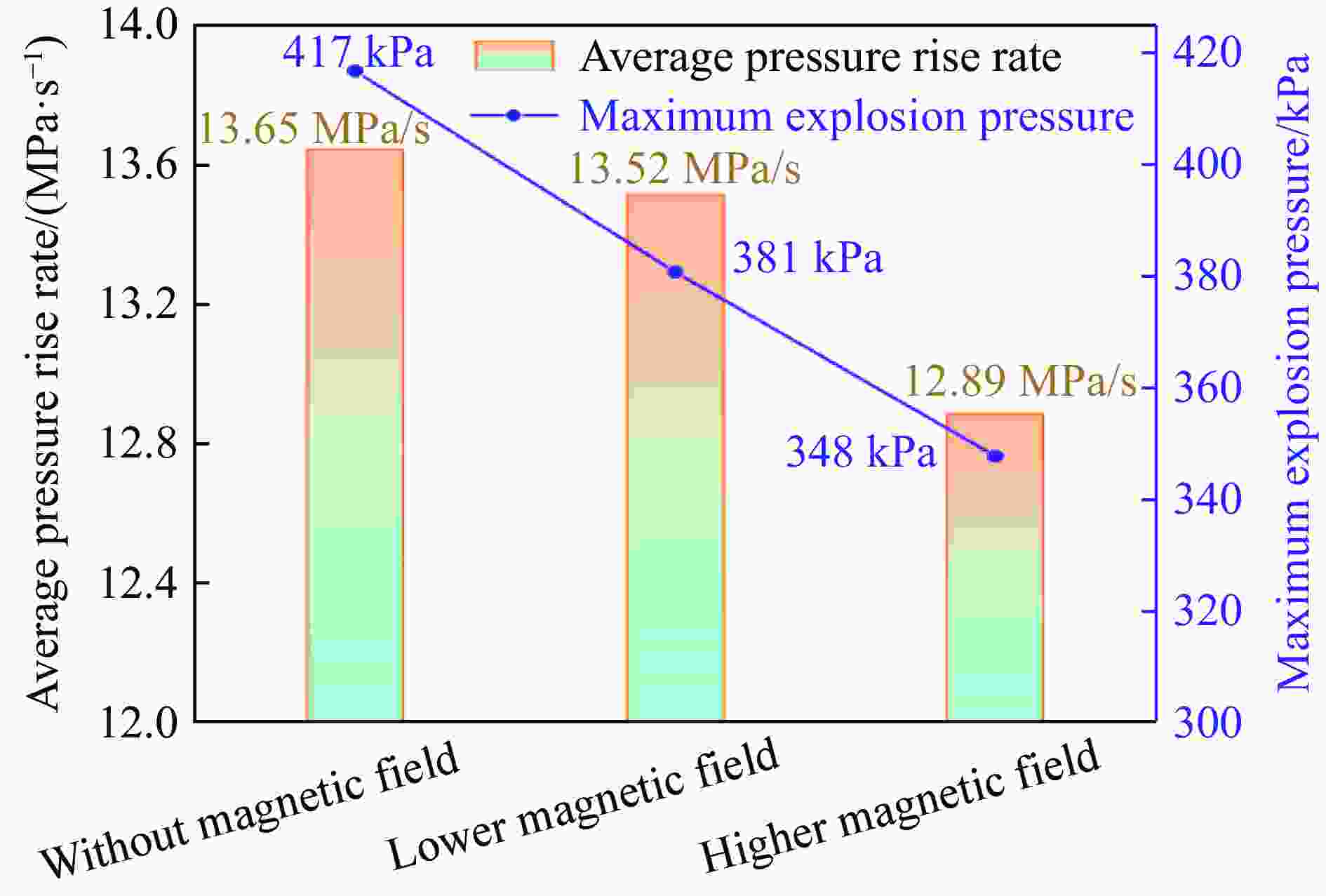
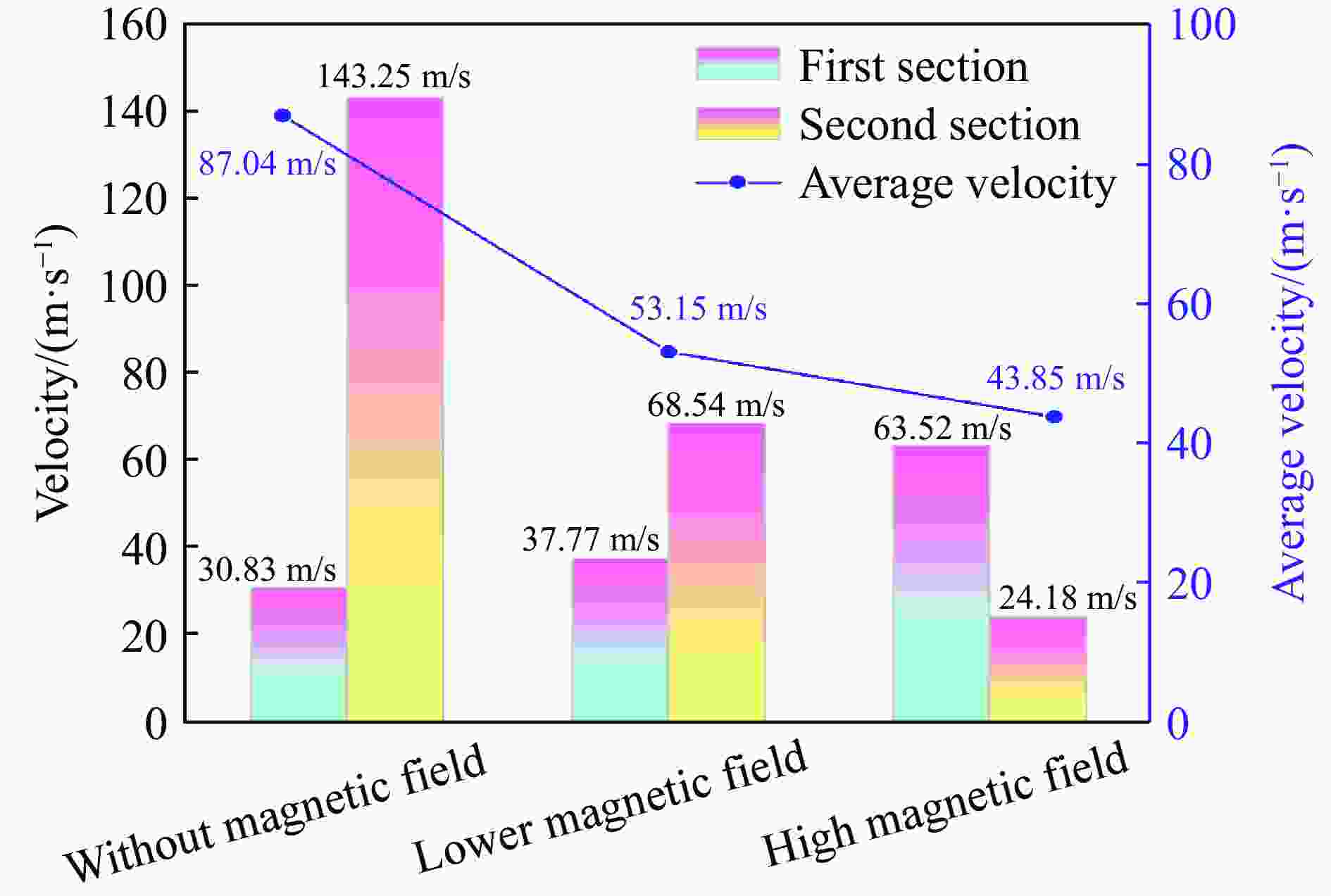
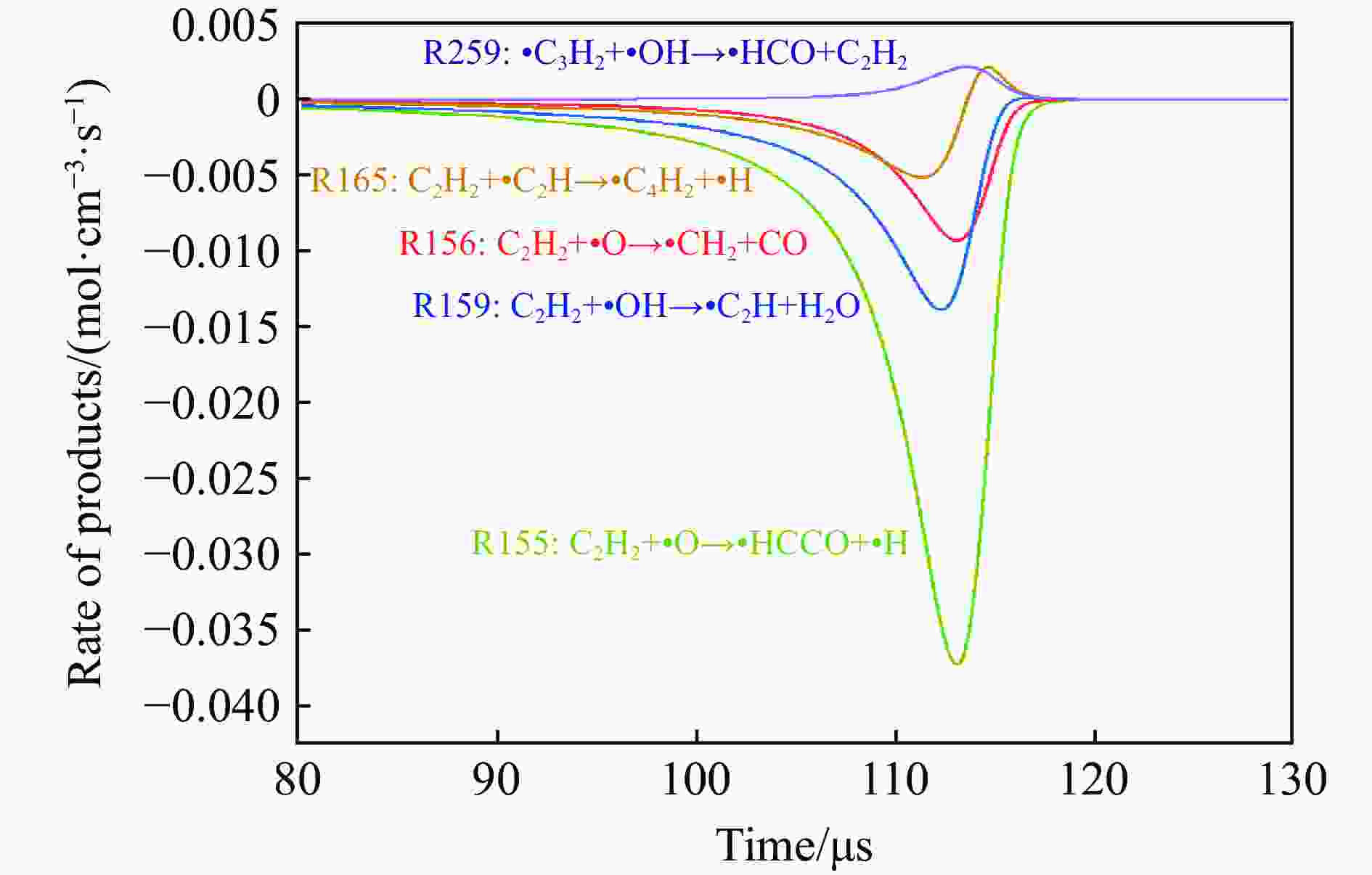
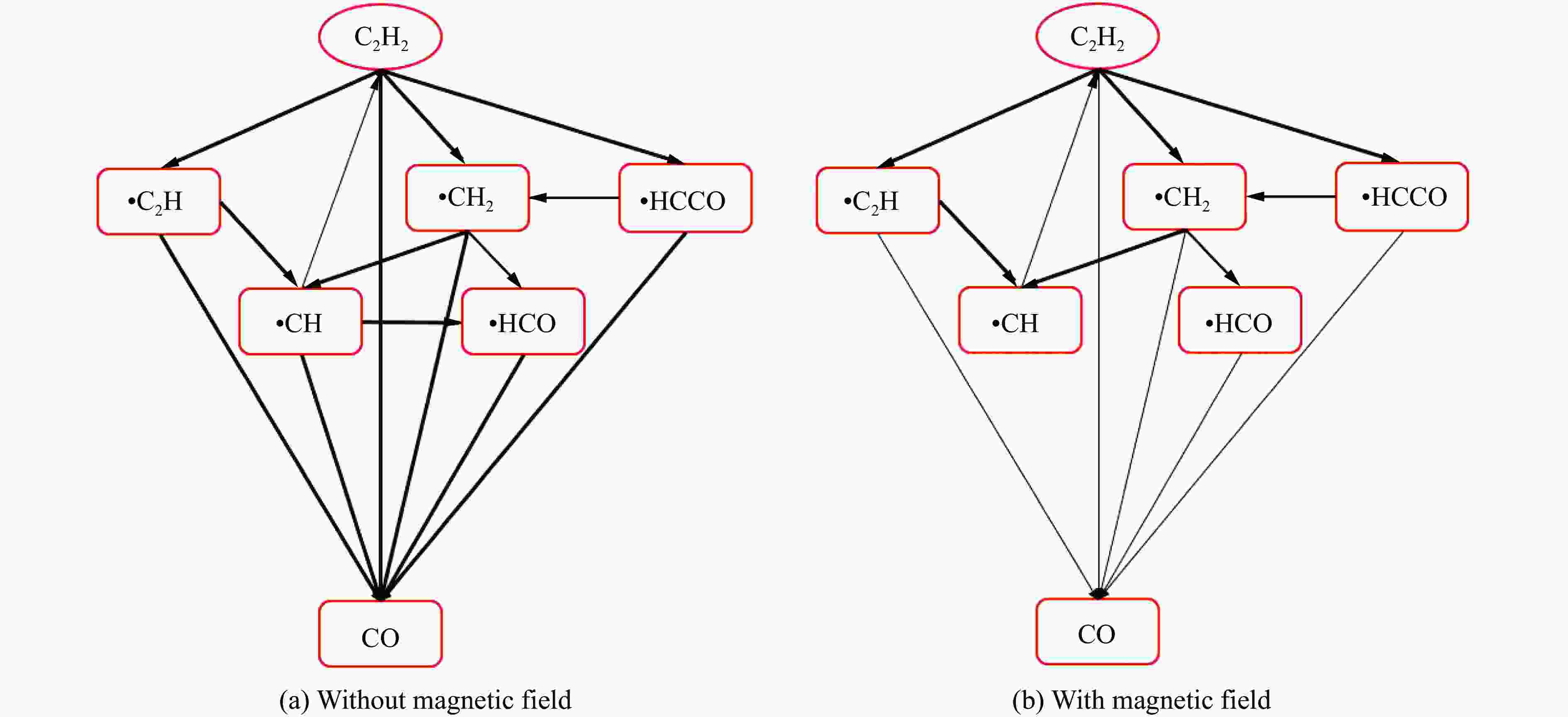
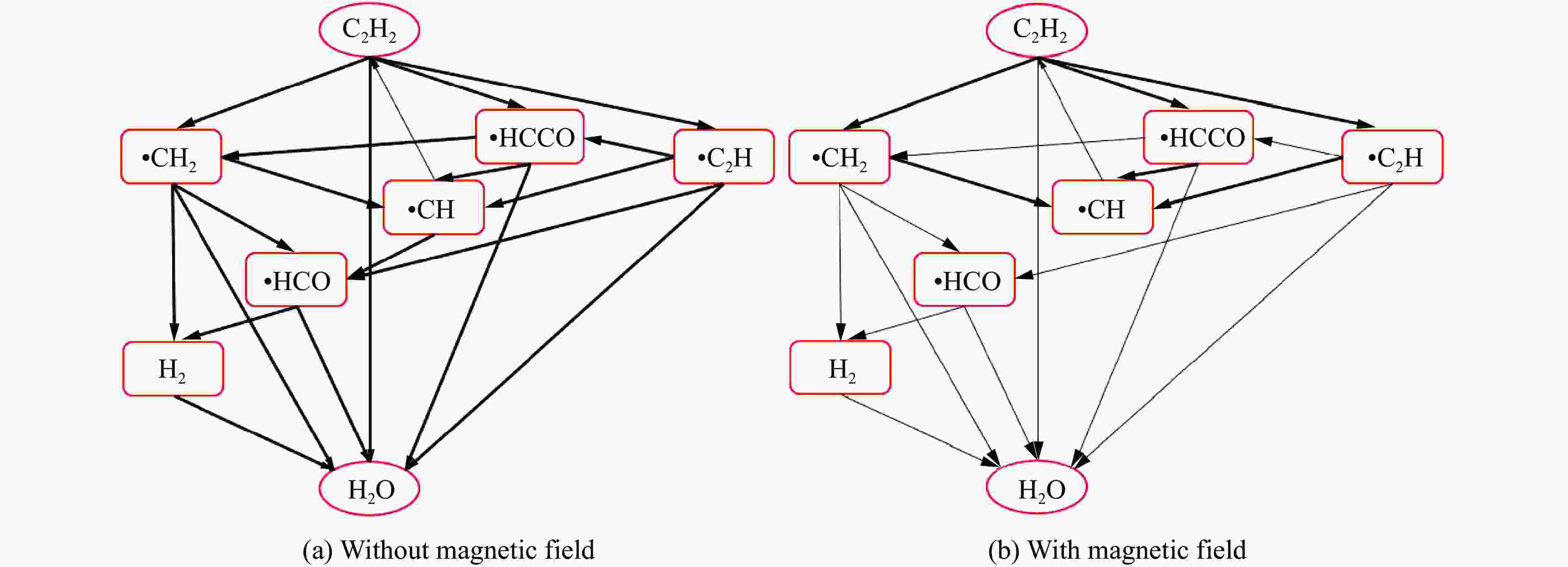
摘要: 为探究磁场对气体爆炸反应的影响,实验研究了磁场强度对C2H2爆炸特征的影响规律,结果表明:磁场能抑制C2H2爆炸压力和升压速率,磁场强度越大,抑制效果越明显;沿火焰传播方向,磁场对C2H2爆炸火焰传播速度呈现先促进后抑制的效果,整体表现为抑制作用。磁场强度较低时,爆炸火焰平均传播速度降低了38.94%,磁场强度较高时,爆炸火焰平均传播速度降低了49.62%。利用Chemkin-Pro软件模拟了C2H2爆炸基元反应过程,理论推导了磁场影响C2H2爆炸的反应机理,磁场改变了C2H2爆炸反应路径,是造成爆炸特征参数下降的主要原因。由于不同种类自由基的摩尔质量和磁化强度不同,在磁场中,洛伦兹力和梯度磁场力对小分子量自由基比对大分子量自由基的作用力更大。磁场改变了自由基的运动轨迹,由于同种小分子量自由基的聚集和器壁效应的产生,减小了关键自由基之间的碰撞几率,降低了基元反应的速率,导致爆炸强度下降。Abstract: To study the effects of magnetic fields on the gas explosion, considering equivalent acetylene premixed combustible gas as the research object, the effects of different magnetic field intensities on acetylene explosion characteristics were studied experimentally. The explosion pressure and flame propagation velocity were measured simultaneously by transient pressure sensors and a detonation velocity instrument, respectively. The results show that the magnetic fields reduce the explosion pressure and the pressure rise rate of acetylene. With increasing magnetic field intensity, the suppression effect is more significant. Along the direction of flame propagation, the magnetic fields first promote and then suppress the explosion flame propagation velocity of acetylene, and the inhibition effect is stronger than the promotion effect. In these experimental conditions, the average propagation velocity of the explosion flame decreased by 38.94% under lower magnetic fields intensity, and at higher magnetic fields intensity, it decreased by 49.62%. To further study the impact mechanism of magnetic fields on premixed combustible gas explosion, the acetylene explosion free radicals reaction process was simulated numerically by Chemkin-Pro software. The chain reactions, rate of products, and sensitivity are analyzed. And the key radical and reaction paths of acetylene explosion are obtained. Combined with the force analysis of magnetic fields on free radicals, it is deduced that magnetic fields change the reaction paths of acetylene to produce carbon dioxide and water, which is the main internal reason for the decrease in explosion parameters. The different free radicals have different molar masses and magnetization. Lorentz force and gradient magnetic field force have stronger effects on small molecular weight free radicals than on large molecular weight free radicals. The calculation shows that the magnetic fields change the trajectory of the free radicals, cause the aggregation of free radicals with the same small molecular weight, and produce a wall effect, which reduces collisions between key free radicals and the rate of elementary reactions, resulting in a decrease of explosion intensity.
-
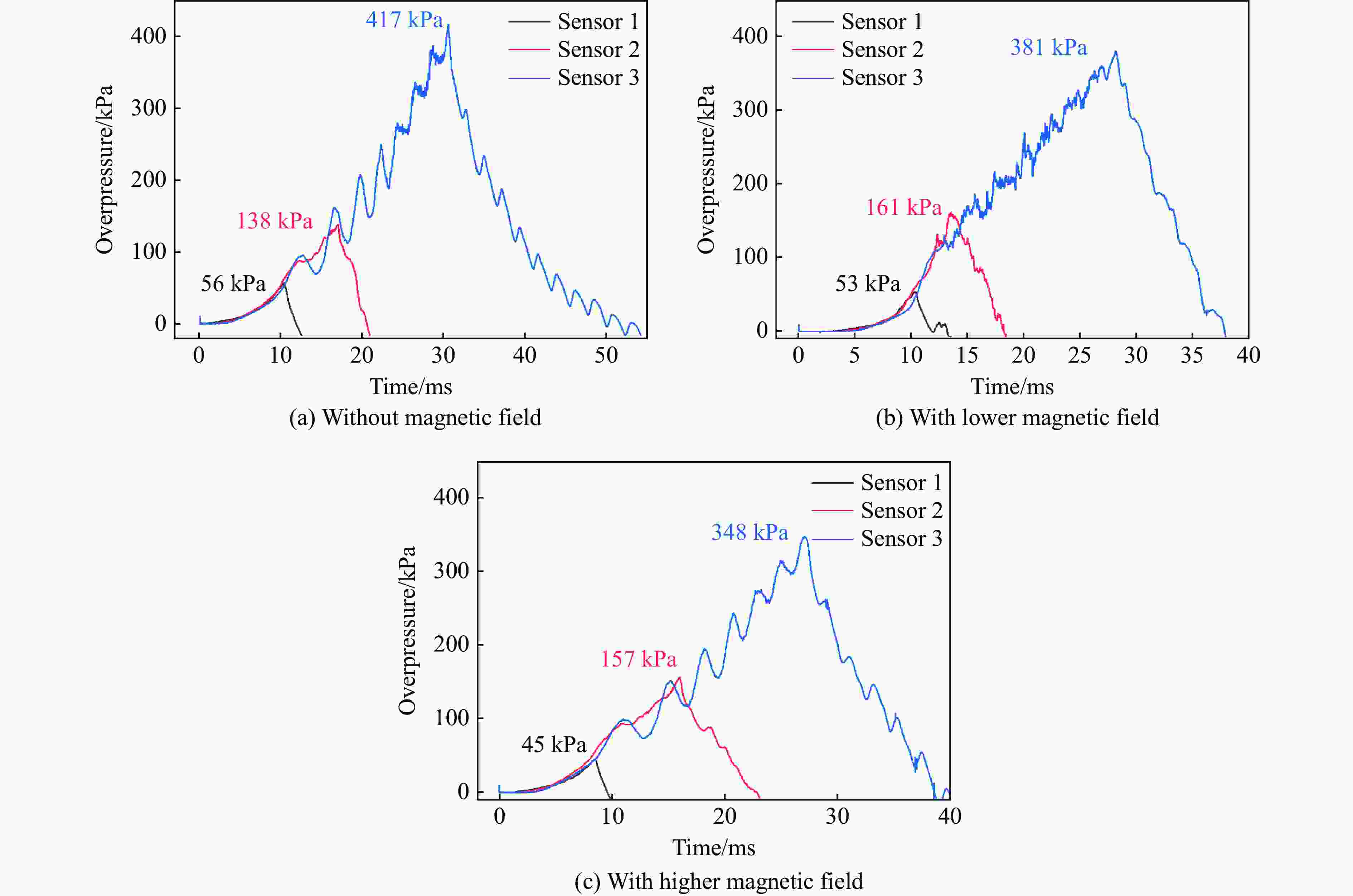
表 1 无磁场时乙炔/空气的爆炸火焰传播速度
Table 1. Flame propagation velocity of C2H2/air explosion without a magnetic field
实验 光纤传感器 距离/mm 时间/μs 速度/(m·s−1) 平均速度/(m·s−1) 1 1~2 300 9945.93 30.16 81.64 2~3 300 2253.58 133.12 2 1~2 300 9893.27 30.32 80.97 2~3 300 2279.23 131.62 3 1~2 300 9369.14 32.02 98.51 2~3 300 1818.18 165.00 表 2 较低磁场强度下乙炔/空气的爆炸火焰传播速度
Table 2. Flame propagation velocity of C2H2/air explosion under lower magnetic field strength
实验 光纤传感器 距离/mm 时间/μs 速度/(m·s−1) 平均速度/(m·s−1) 1 1~2 300 7955.45 37.71 53.02 2~3 300 4390.46 68.33 2 1~2 300 7874.02 38.10 52.80 2~3 300 4444.26 67.50 3 1~2 300 8002.13 37.49 53.64 2~3 300 4298.61 69.79 表 3 较高磁场强度下乙炔/空气的爆炸火焰传播速度
Table 3. Flame propagation velocity of C2H2/air explosion under higher magnetic field strength
实验 光纤传感器 距离/mm 时间/μs 速度/(m·s−1) 平均速度/(m·s−1) 1 1~2 300 4917.23 61.01 41.81 2~3 300 13269.07 22.61 2 1~2 300 4705.88 63.75 43.88 2~3 300 12494.15 24.01 3 1~2 300 4558.58 65.81 45.86 2~3 300 11577.70 25.91 表 4 起始参数
Table 4. Initial parameters
C2H2体积分数/% N2体积分数/% O2体积分数/% 温度/K 压力/kPa 时间/s 7.7 72.917 19.383 1200 101 0.05 -
[1] REVANTH A V, MALAIKANNAN G, MALHOTRA V. On the effect of repulsive magnetic field on partially premixed flames [J]. IOP Conference Series:Materials Science and Engineering, 2020, 912(4): 042020. DOI: 10.1088/1757-899X/912/4/042020. [2] OOMMEN L P, NARAYANAPPA K G, VIJAYALAKSHMI S K. Experimental analysis of synergetic effect of part-cooled exhaust gas recirculation on magnetic field-assisted combustion of liquefied petroleum gas [J]. Arabian Journal for Science and Engineering, 2020, 45(11): 9187–9196. DOI: 10.1007/s13369-020-04696-z. [3] KUMAR M, AGARWAL S, KUMAR V, et al. Experimental investigation on butane diffusion flames under the influence of magnetic field by using digital speckle pattern interferometry [J]. Applied Optics, 2015, 54(9): 2450–2460. DOI: 10.1364/AO.54.002450. [4] AGARWAL S, KUMAR M, SHAKHER C. Experimental investigation of the effect of magnetic field on temperature and temperature profile of diffusion flame using circular grating Talbot interferometer [J]. Optics and Lasers in Engineering, 2015, 68: 214–221. DOI: 10.1016/J.OPTLASENG.2015.01.004. [5] ITOH S, SHINODA M, KITAGAWA K, et al. Spatially resolved elemental analysis of a hydrogen-air diffusion flame by laser-induced plasma spectroscopy (LIPS) [J]. Microchemical Journal, 2001, 70(2): 143–152. DOI: 10.1016/S0026-265X(01)00107-2. [6] KAJIMOTO T, YAMADA E, SHINODA M, et al. Dependence of magnetically induced change in oh distribution in a methane-air premixed flame on equivalence ratio [J]. Combustion Science and Technology, 2003, 175(9): 1611–1623. DOI: 10.1080/00102200302369. [7] SHINODA M, YAMADA E, KAJIMOTO T, et al. Mechanism of magnetic field effect on OH density distribution in a methane–air premixed jet flame [J]. Proceedings of the Combustion Institute, 2005, 30(1): 277–284. DOI: 10.1016/J.PROCI.2004.07.006. [8] YAMADA E, KITABAYASHI N, HAYASHI A K, et al. Mechanism of high-pressure hydrogen auto-ignition when spouting into air [J]. International Journal of Hydrogen Energy, 2011, 36(3): 2560–2566. DOI: 10.1016/J.IJHYDENE.2010.05.011. [9] YAMADA E, SHINODA M, YAMASHITA H, et al. Experimental and numerical analyses of magnetic effect on OH radical distribution in a hydrogen-oxygen diffusion flame [J]. Combustion and Flame, 2003, 135(4): 365–379. DOI: 10.1016/J.COMBUSTFLAME.2003.08.005. [10] YAMADA E, SHINODA M, YAMASHITA H, et al. Numerical analysis of a hydrogen-oxygen diffusion flame in vertical or horizontal gradient of magnetic field [J]. Combustion Science and Technology, 2002, 174(9): 149–164. DOI: 10.1080/713713079. [11] YAMADA E, SHINODA M, YAMASHITA H, et al. Influence of four kinds of gradient magnetic fields on hydrogen-oxygen flame [J]. AIAA Journal, 2003, 41(8): 1535–1541. DOI: 10.2514/2.2104. [12] 高建村, 王乐, 胡守涛, 等. 不同磁性金属丝对丙烷爆炸反应抑制机理研究 [J]. 中国安全生产科学技术, 2020, 16(7): 125–130. DOI: 10.11731/j.issn.1673-193x.2020.07.020.GAO J C, WANG L, HU S T, et al. Study on inhibition mechanism of different magnetic metal wires on propane explosion [J]. Journal of Safety Science and Technology, 2020, 16(7): 125–130. DOI: 10.11731/j.issn.1673-193x.2020.07.020. [13] ZHOU S Y, GAO J C, LUO Z M, et al. Effects of mesh aluminium alloy and aluminium velvet on the explosion of H2/air, CH4/air and C2H2/air mixtures [J]. International Journal of Hydrogen Energy, 2021, 46(27): 14871–14880. DOI: 10.1016/J.IJHYDENE.2021.01.200. [14] 左俊祥. 反应体系HCl+OH与O+C2H2的势能面及动力学理论研究[D]. 南京: 南京大学, 2019.ZUO J X. Theoretical studies of potential energy surfaces and dynamics for the HCl+OH and O+C2H2 reaction systems[D]. Nanjing: Nanjing University, 2019. [15] BASTIN E, DELFAU J L, REUILLON M, et al. Experimental and computational investigation of the structure of a sooting C2H2-O2-Ar flame [J]. Symposium (International) on Combustion, 1989, 22(1): 313–322. DOI: 10.1016/S0082-0784(89)80037-2. [16] WINTER J, BERNDT J, HONG S H, et al. Dust formation in Ar/CH4 and Ar/C2H2 plasmas [J]. Plasma Sources Science and Technology, 2009, 18(3): 034010. DOI: 10.1088/0963-0252/18/3/034010. [17] SANDER R K, TIEE J J, QUICK C R, et al. Quenching of C2H emission produced by vacuum ultraviolet photolysis of acetylene [J]. The Journal of Chemical Physics, 1988, 89(6): 3495–3501. DOI: 10.1063/1.454920. [18] MCKEE K W, BLITZ M A, CLEARY P A, et al. Experimental and master equation study of the kinetics of OH+C2H2: temperature dependence of the limiting high pressure and pressure dependent rate coefficients [J]. The Journal of Physical Chemistry A, 2007, 111(19): 4043–4055. DOI: 10.1021/JP067594Y. [19] SMITH I W M, ZELLNE R. Rate measurements of reactions of OH by resonance absorption. Part 2. —Reactions of OH with CO, C2H4 and C2H2 [J]. Journal of the Chemical Society, Faraday Transactions2:Molecular and Chemical Physics, 1973, 69: 1617–1627. DOI: 10.1039/F29736901617. [20] HIRAOKA K, TAKAYAMA T, EUCHI A, et al. Study of the reactions of H and D atoms with solid C2H2, C2H4, and C2H6 at cryogenic temperatures [J]. The Astrophysical Journal, 2000, 532(2): 1029–1037. DOI: 10.1086/308612. [21] YANG X G, HU S T, WANG L, et al. Effect of magnetic field on dynamics of 5% propane/air premixed gases [J]. Journal of Physics:Conference Series, 2021, 1948: 012133. DOI: 10.1088/1742-6596/1948/1/012133. [22] WANG H, YOU X Q, JOSHI AV, et al. USC mech version II. High-temperature combustion reaction model of H2/CO/C1-C4 compounds[DB/OL]. http://ignis.usc.edu/USC_Mech_II.htm.2007. [23] OOMMEN L P, KUMAR G N. A study on the effect of magnetic field on the properties and combustion of hydrocarbon fuels [J]. International Journal of Mechanical and Production Engineering Research and Development (IJMPERD), 2019, 9(3): 89–98. DOI: 10.24247/IJMPERDJUN20199. [24] AOKI T. Radical emissions and butane diffusion flames exposed to uniform magnetic fields encircled by magnetic gradient fields [J]. Japanese Journal of Applied Physics, 1990, 29(5R): 952–957. DOI: 10.1143/JJAP.29.952. [25] MIZUTANI Y, FUCHIHATA M, OHKURA Y. Pre-mixed laminar flames in a uniform magnetic field [J]. Combustion and flame, 2001, 125(1/2): 1071–1073. DOI: 10.1016/S0010-2180(00)00244-3. -






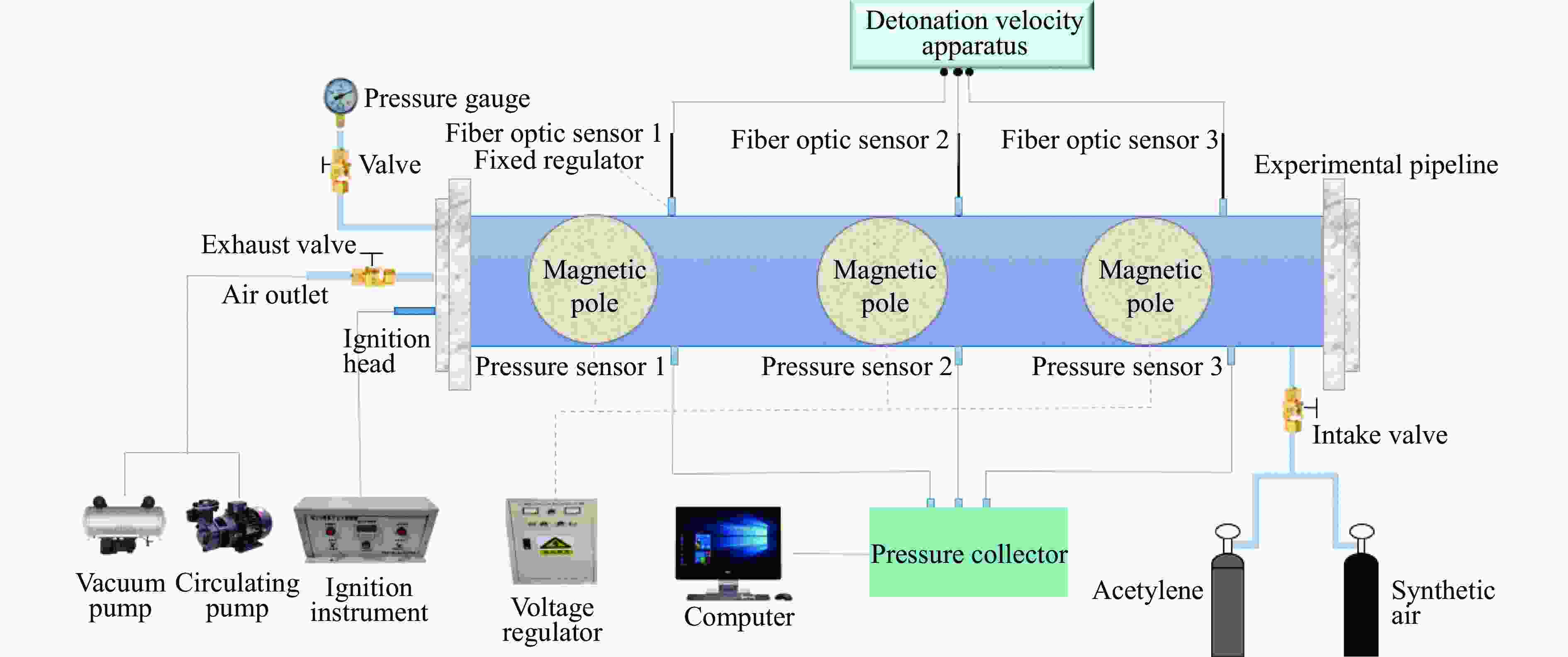
 下载:
下载: