The upper explosion limit of C3H8/C2H4 mixtures in air at high temperatures and pressures
-
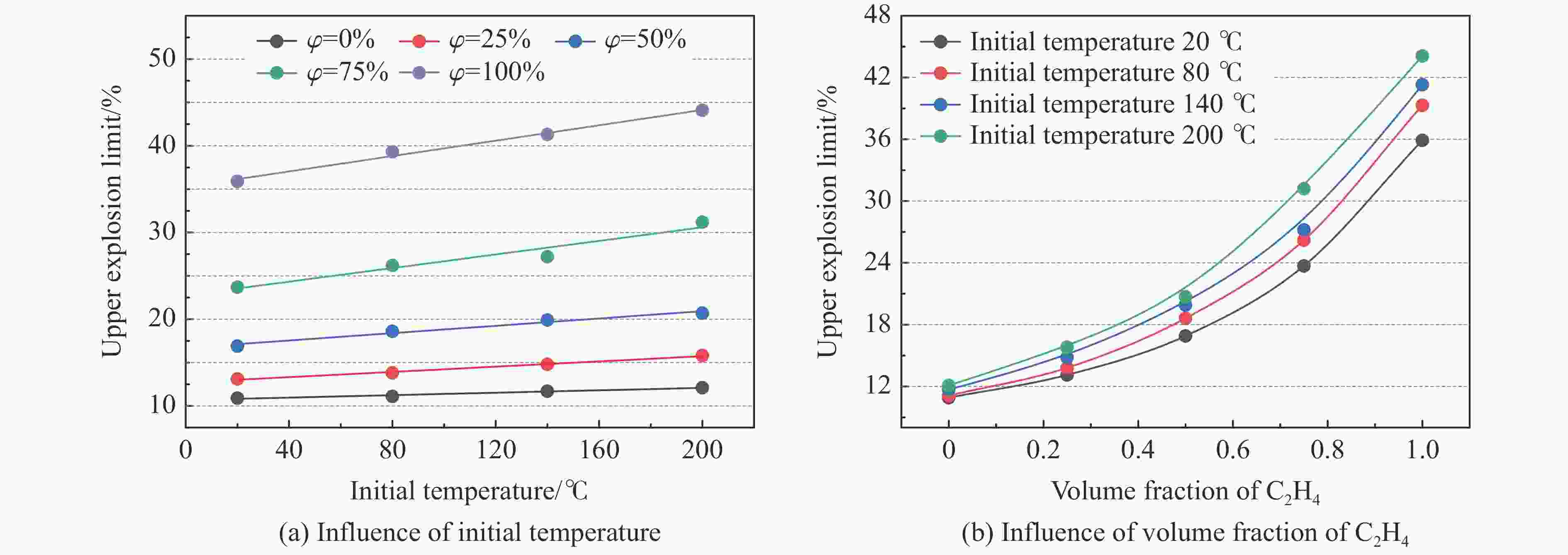
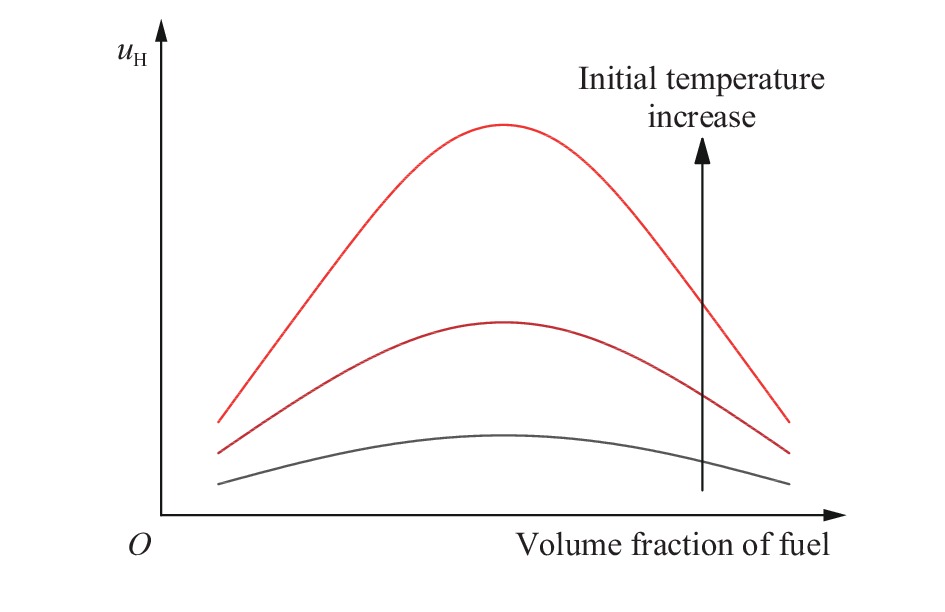
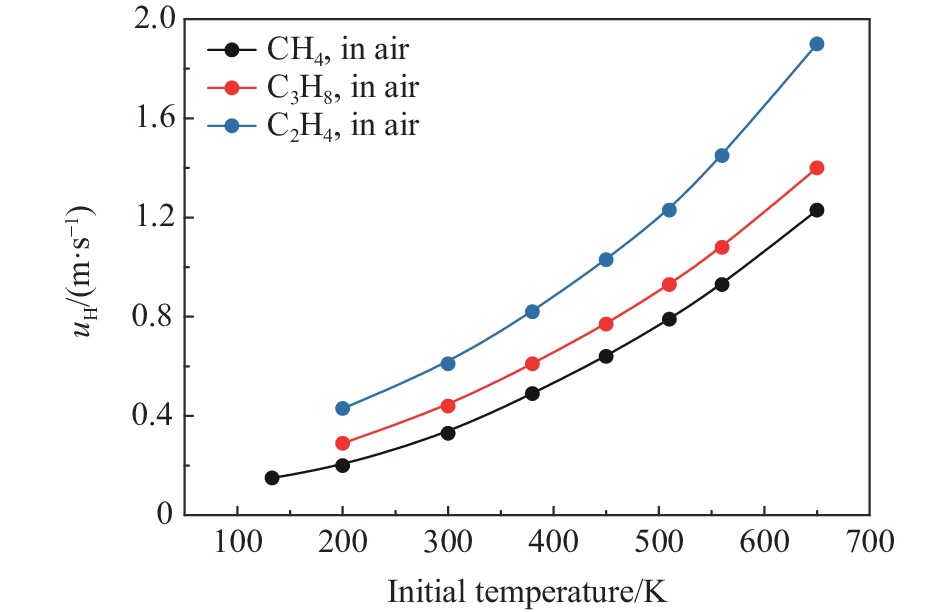
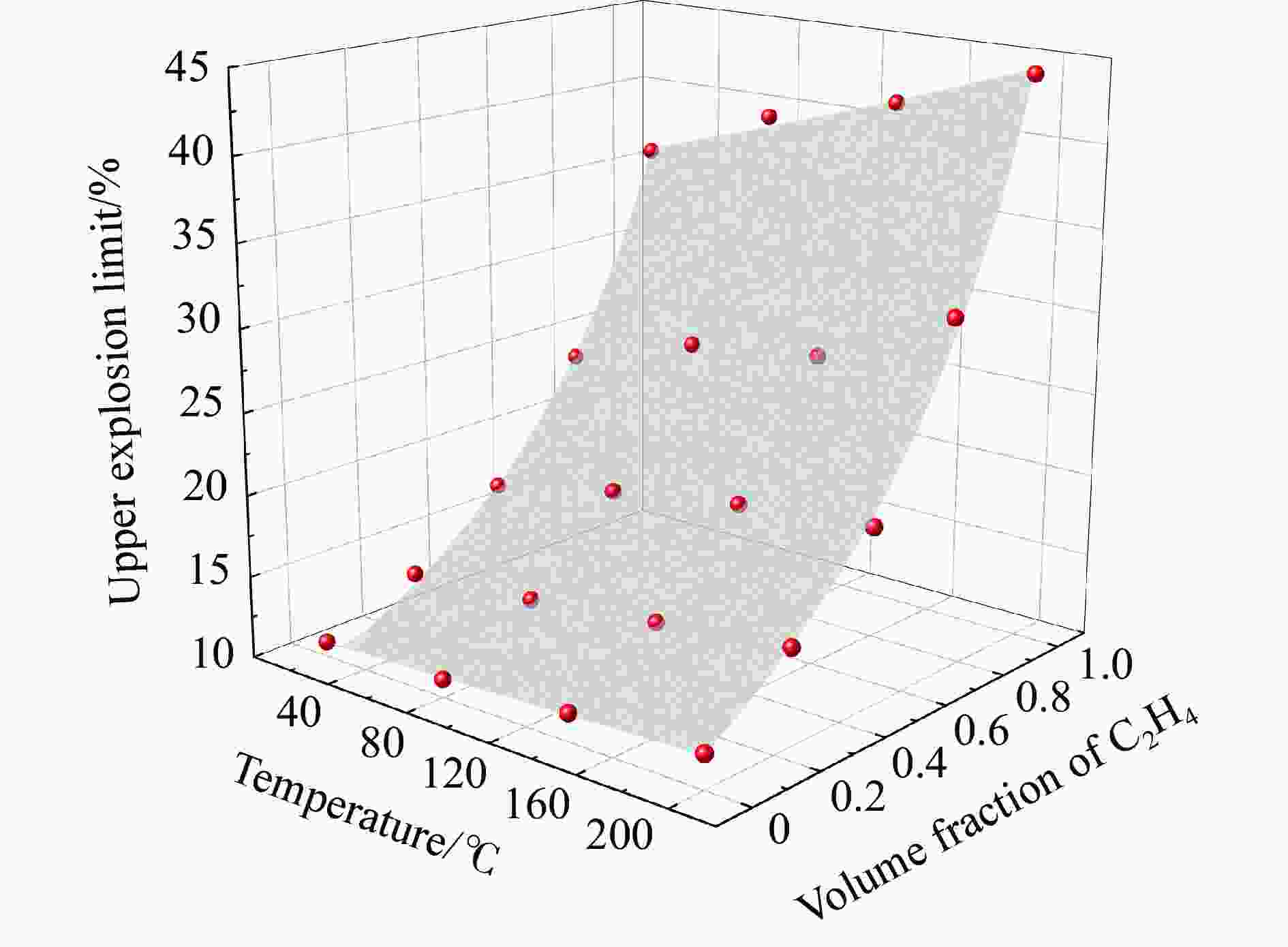
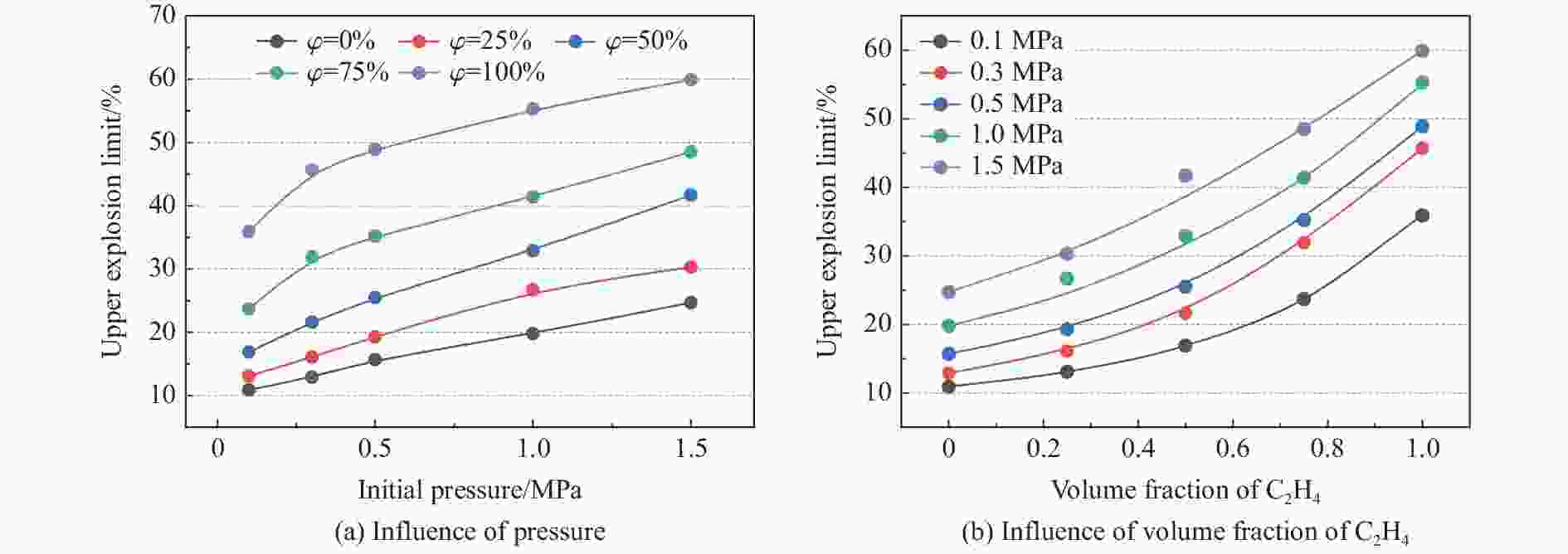
摘要: 为了防控高温高压工艺流程中可燃混合气体潜在的爆炸风险,利用自行搭建的20 L球形爆炸特性实验装置,测试了初始温度20~200 ℃、初始压力0.1~1.5 MPa下C3H8/C2H4混合气体在空气中的爆炸上限,分析了温度、压力和C2H4体积分数对混合气体爆炸上限的影响。结果表明,随着温度和压力的升高,C3H8/C2H4混合气体爆炸上限升高。当初始压力高于0.3 MPa时,随着C2H4体积分数的增加,爆炸上限的上升速率明显降低。随着C2H4体积分数的增加,高温和高压下爆炸上限的提升幅度和速率比常温常压下更高。温度和压力的协同作用对爆炸上限的影响远大于二者单独作用的影响之和,即高温和高压协同作用下,C3H8/C2H4混合气体具有更高的爆炸风险,且随着C2H4体积分数的增加,爆炸风险会进一步提升。分别拟合得到了爆炸上限与温度参数、爆炸上限与压力参数以及爆炸上限与温度和压力双参数下的函数关系。
-
关键词:
- 高温 /
- 高压 /
- 爆炸上限 /
- C3H8/C2H4混合气体
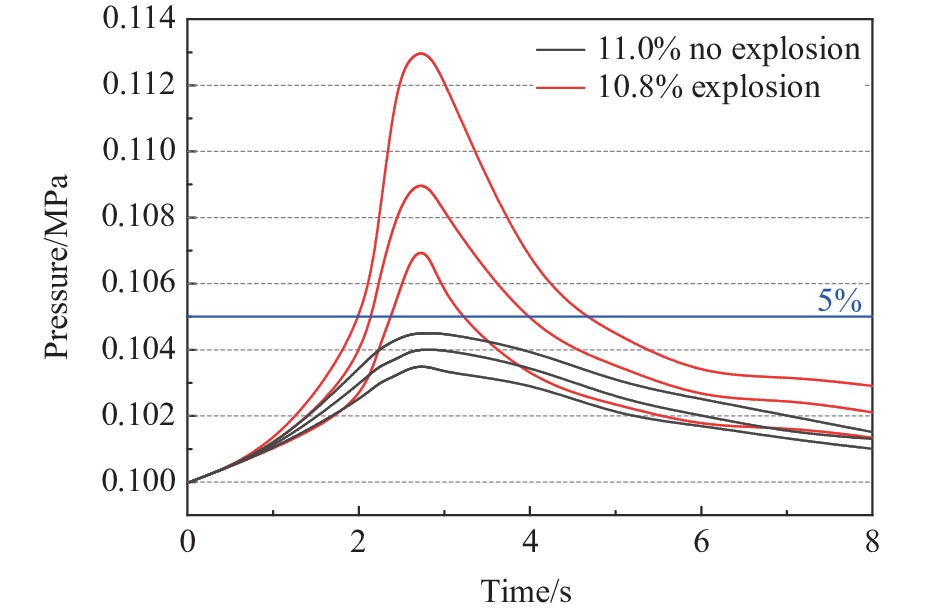
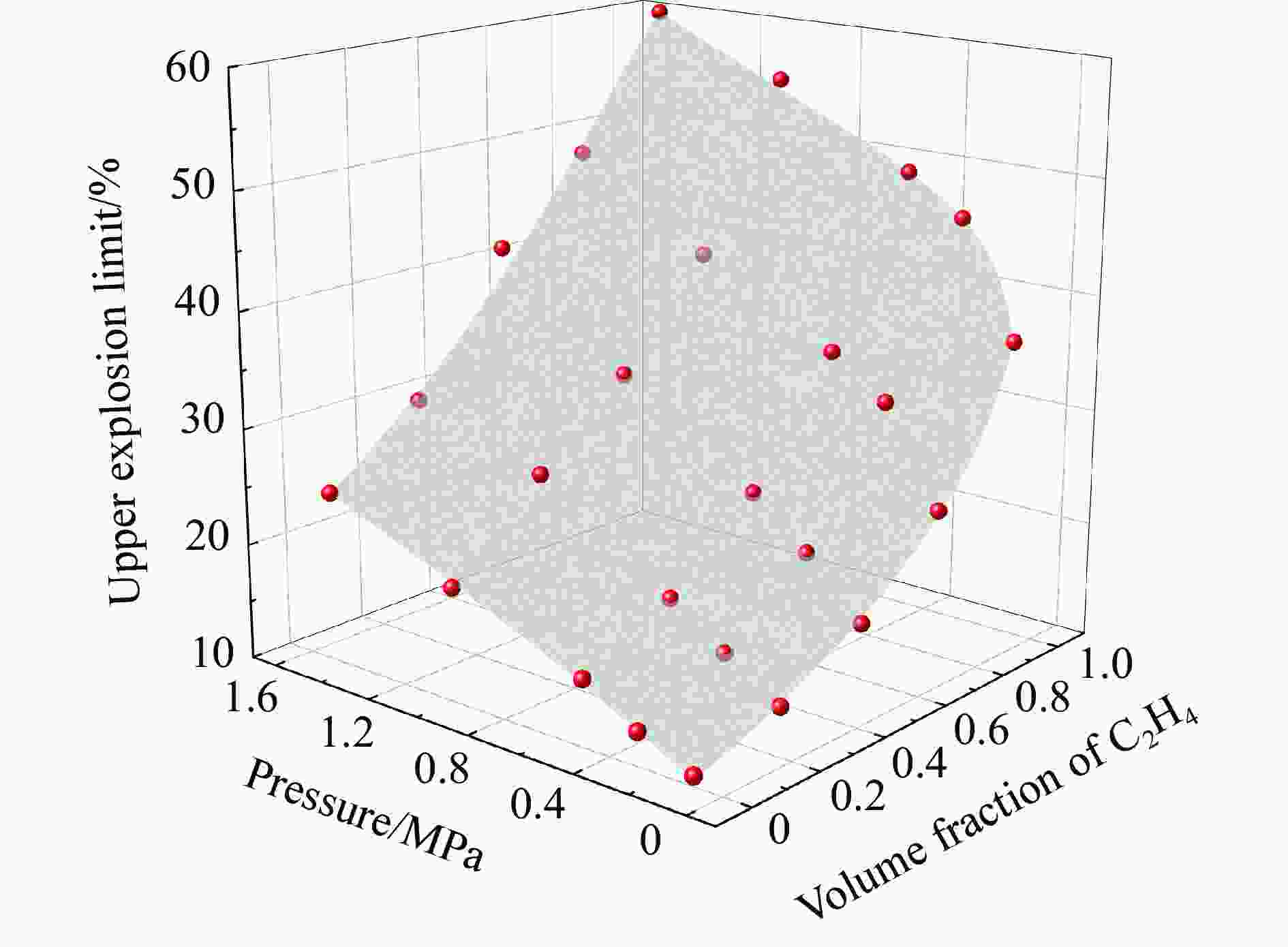
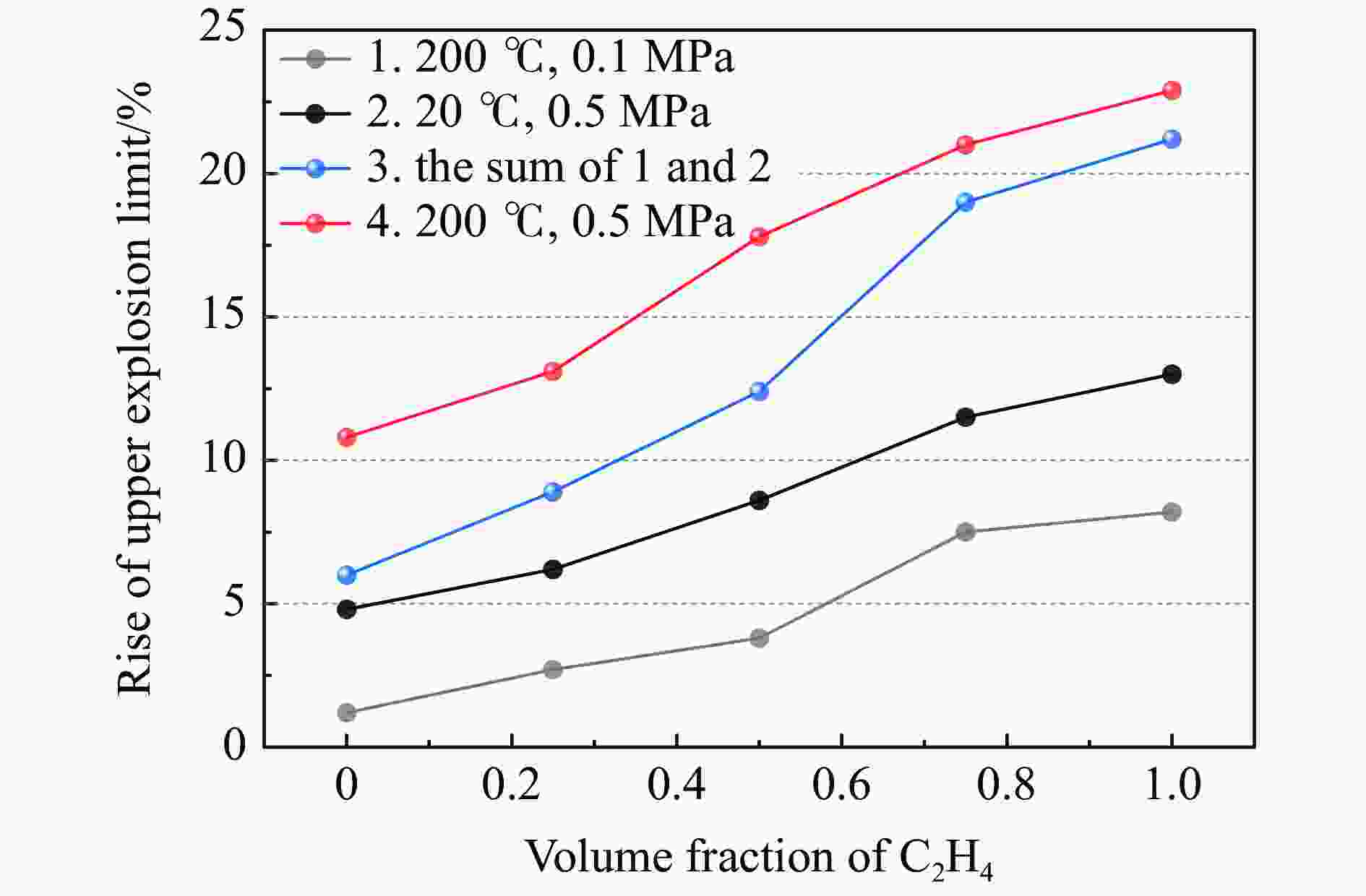
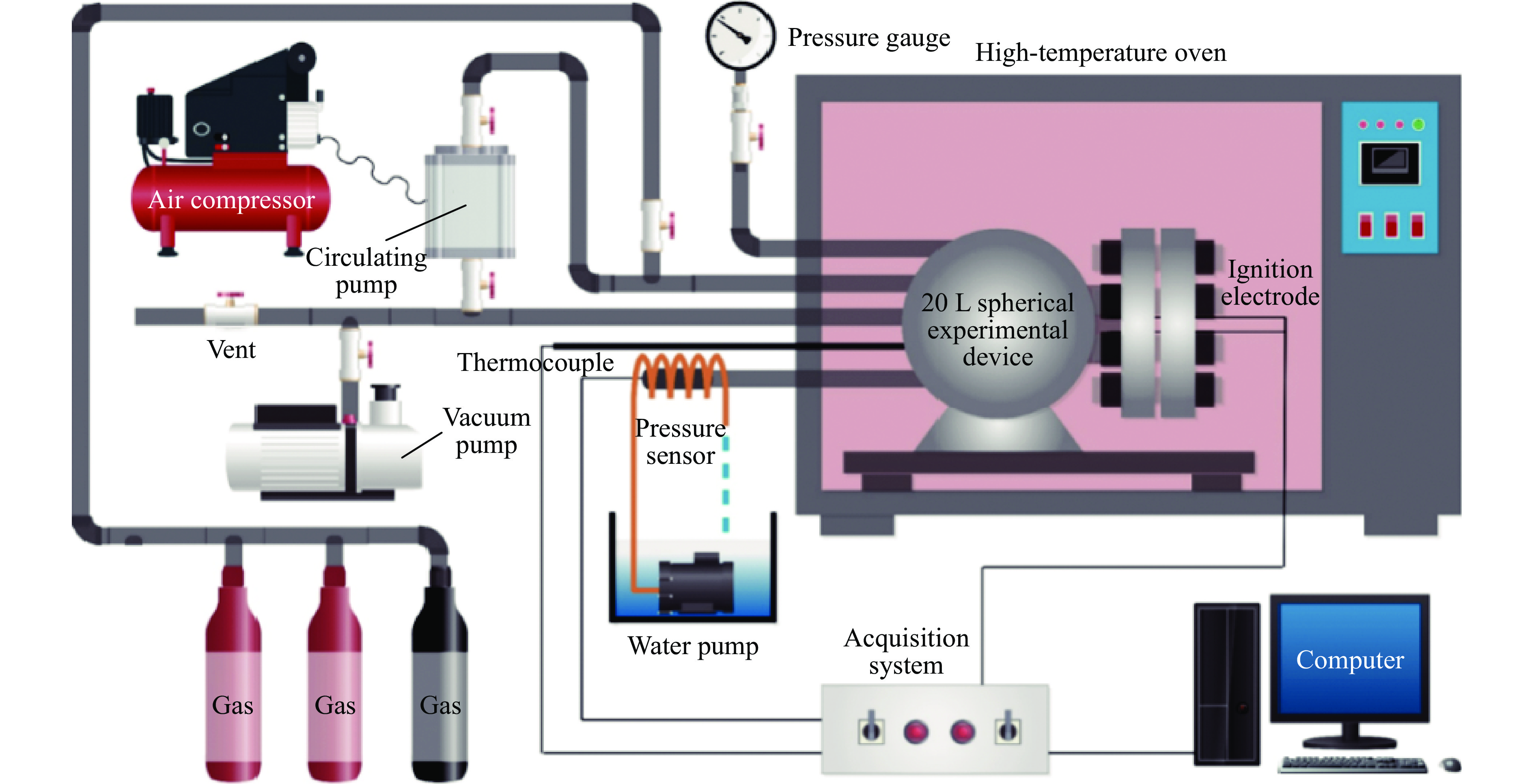
Abstract: It is necessary to understand the upper explosion limits of C3H8/C2H4 mixtures to prevent the potential explosive risks of flammable gas mixtures in the process of high temperatures and pressures. An experimental device of a 20 L spherical vessel with high pressure placed in a high-temperature oven was set up to test the upper explosion limits of C3H8/C2H4 mixtures at high pressure and temperature. The partial pressure method was used to prepare the mixtures of C3H8, C2H4, and air with a certain concentration. A pressure rise amplitude of 5% was adopted to judge whether the explosion occurred. The initial temperature ranged from 20 ℃ to 200 ℃, and the initial pressure ranged from 0.1 MPa to 1.5 MPa in the experiments. The effects of temperature, pressure, and volume fraction of C2H4 on the upper explosion limit of C3H8/C2H4 mixtures were analyzed. The results show that the upper explosion limit of C3H8/C2H4 mixtures increases with the rises of temperature and pressure, but the increase rate of the upper explosion limit decreases significantly with the increase of C2H4 concentration when the initial pressure is higher than 0.3 MPa. The amplitude increase and rate of the upper explosion limit with C2H4 at high temperatures and pressures are higher than those at normal conditions. The influences of temperature and pressure on the upper explosion limit are much greater than the sum of the two effects alone, indicating that the C3H8/C2H4 mixtures have a higher explosion risk under the synergistic effect of high temperature and pressure, and it will be further enhanced with the increase of C2H4 concentration. The influence of the temperature, pressure, and their synergistic effects on the upper explosion limit of C3H8/C2H4 mixtures in different proportions are comprehensively analyzed, and the corresponding functional relations of the temperature-upper explosion limit, pressure-upper explosion limit, and temperature-pressure-upper explosion limit in different volume fractions of C2H4 are summarized by the non-linear regression of surface.-
Key words:
- high temperature /
- high pressure /
- upper explosion limit /
- C3H8/C2H4 mixture
-
表 1 气体爆炸上限
Table 1. The upper explosion limits of gases
表 2 式(1)中的拟合参数
Table 2. Dimensionless fitting coefficients of Eq. (1)
${ {U_{0}} }$ ${A_{01}}$ ${B_{01}}$ ${B_{02}}$ ${ {C_{1}} }$ ${A_{1}}$ ${A_{2}}$ ${B_{1}}$ ${B_{2}}$ ${C_{2}}$ R2 17.137 20.458 11392.409 7390.431 31.393 1.922 −0.001 1300.26 −767.583 −1.097 0.99 表 3 式(2)中的拟合参数
Table 3. Dimensionless fitting coefficients of Eq. (2)
${{U_{0}^\prime}} $ ${A_{01}^\prime} $ ${B_{01}^\prime} $ ${B_{02}^\prime} $ ${{C_{1}^\prime}} $ ${{A_{1}^\prime}} $ ${{A_{2}^\prime}} $ ${{B_{1}^\prime}} $ ${{B_{2}^\prime}} $ ${{C_{2}^\prime}} $ R2 9.043 17.596 −5.211 −4.274 0.346 0.372 −0.054 −1.495 0.495 0.002 0.99 表 4 式(3)~(4)中的拟合参数
Table 4. Dimensionless fitting coefficients of Eqs. (3)−(4)
$\varphi $/% A B C D E F R2 0 16.49393 2.562 46×10−4 2.10108 2.53202 1.031 61×10−4 − 0.98 25 −23.80212 −0.01601 1.59727 39.67630 2.41452 0.01812 0.99 50 27.82329 0.00662 1.54223 7.82659 0.14891 0.00388 0.99 75 39.82627 0.00669 1.52933 8.21195 0.04068 0.00230 0.99 100 52.12125 0.03811 1.17643 5.65889 −0.04521 0.00729 0.99 -
[1] BOUNACEUR R, GLAUDE P A, SIRJEAN B, et al. Prediction of flammability limits of gas mixtures containing inert gases under variable temperature and pressure conditions [C] // ASME Turbo Expo 2017: Turbomachinery Technical Conference and Exposition. Charlotte, France, 2017. [2] LIAW H J, CHEN K Y. A model for predicting temperature effect on flammability limits [J]. Fuel, 2016, 178: 179–187. DOI: 10.1016/j.fuel.2016.03.034. [3] VAN DEN SCHOOR F, HERMANNS R T E, VAN OIJEN J A, et al. Comparison and evaluation of methods for the determination of flammability limits, applied to methane/hydrogen/air mixtures [J]. Journal of Hazardous Materials, 2008, 150(3): 573–581. DOI: 10.1016/j.jhazmat.2007.05.006. [4] VAN DEN SCHOOR F, VERPLAETSEN F. The upper explosion limit of lower alkanes and alkenes in air at elevated pressures and temperatures [J]. Journal of Hazardous Materials, 2006, 128(1): 1–9. DOI: 10.1016/j.jhazmat.2005.06.043. [5] 李刚, 李玉峰, 苑春苗. 高温和高压下CBM的爆炸极限 [J]. 东北大学学报(自然科学版), 2012, 33(4): 580–583. DOI: 10.12068/j.issn.1005-3026.2012.04.030.LI G, LI Y F, YUAN C M. Explosion limits of CBM at elevated pressure and temperature [J]. Journal of Northeastern University Natural Science, 2012, 33(4): 580–583. DOI: 10.12068/j.issn.1005-3026.2012.04.030. [6] HUANG L, LI Z, WANG Y, et al. Experimental assessment on the explosion pressure of CH4-air mixtures at flammability limits under high pressure and temperature conditions [J]. Fuel, 2021, 299: 120868. DOI: 10.1016/j.fuel.2021.120868. [7] WANG Y L, QI C, NING Y, et al. Experimental determination of the lower flammability limit and limiting oxygen concentration of propanal/air mixtures under elevated temperatures and pressures [J]. Fuel, 2022, 326: 124882. DOI: 10.1016/j.fuel.2022.124882. [8] WANG Y L, YU J L, YAN X Q, et al. Study on the explosion characteristics of propanal/air mixtures at elevated pressures [J]. Fuel, 2022, 328: 125288. DOI: 10.1016/j.fuel.2022.125288. [9] YU X Z, YU J L, JI W T, et al. A research on flammability limits of the refrigerant HCFC-22/air mixtures at elevated pressures [J]. Journal of Loss Prevention in the Process Industries, 2019, 61: 89–93. DOI: 10.1016/j.jlp.2019.05.022. [10] LUO Z M, SU B, WANG T, et al. Effects of propane on the flammability limits and chemical kinetics of methane-air explosions [J]. Combustion Science and Technology, 2020, 192(9): 1785–1801. DOI: 10.1080/00102202.2019.1625041. [11] TONG M, WU G, HAO J, et al. Explosion limits for combustible gases [J]. Mining Science and Technology, 2009, 19(2): 182–184. DOI: 10.3969/j.issn.2095-2686.2009.02.009. [12] 宁也, 何萌, 祁畅, 等. 三元可燃混合气体爆炸极限实验及预测方法 [J]. 爆炸与冲击, 2023, 43(4): 045401. DOI: 10.11883/bzycj-2022-0120.NING Y, HE M, QI C, et al. Experiment and methods of prediction on the explosion limit of the ternary flammable gas mixture [J]. Explosion and Shock Waves, 2023, 43(4): 045401. DOI: 10.11883/bzycj-2022-0120. [13] CUI G, YANG C, LI Z L, et al. Experimental study and theoretical calculation of flammability limits of methane/air mixture at elevated temperatures and pressures [J]. Journal of Loss Prevention in the Process Industries, 2016, 41: 252–258. DOI: 10.1016/j.jlp.2016.02.016. [14] MASHUGA C V, CROWL D A. Flammability zone prediction using calculated adiabatic flame temperatures [J]. Process Safety Progress, 1999, 18(3): 127–134. DOI: 10.1002/prs.680180303. [15] HU X, YU Q, SUN N, et al. Experimental study of flammability limits of oxy-methane mixture and calculation based on thermal theory [J]. International Journal of Hydrogen Energy, 2014, 39(17): 9527–9533. DOI: 10.1016/j.ijhydene.2014.03.202. [16] GIURCAN V, RAZUS D, MITU M, et al. Prediction of flammability limits of fuel-air and fuel-air-inert mixtures from explosivity parameters in closed vessels [J]. Journal of Loss Prevention in the Process Industries, 2015, 34: 65–71. DOI: 10.1016/j.jlp.2015.01.025. [17] QI C, WANG Y L, NING Y, et al. Flammability limits of combustible gases at elevated temperatures and pressures: recent advances and future perspectives [J]. Energy and Fuels, 2022, 36(21): 12896–12916. DOI: 10.1021/acs.energyfuels.2c02567. [18] LIU X, ZHANG Q. Influence of initial pressure and temperature on flammability limits of hydrogen-air [J]. International Journal of Hydrogen Energy, 2014, 39(12): 6774–6782. DOI: 10.1016/j.ijhydene.2014.02.001. [19] YU X Z, YAN X Q, JI W T, et al. Effect of super-ambient conditions on the upper explosion limit of ethane/oxygen and ethylene/oxygen mixtures [J]. Journal of Loss Prevention in the Process Industries, 2019, 59: 100–105. DOI: 10.1016/j.jlp.2019.03.009. [20] 喻健良, 姚福桐, 于小哲, 等. 高温和高压对乙烷在氧气中爆炸极限影响的实验研究 [J]. 爆炸与冲击, 2019, 39(12): 122101. DOI: 10.11883/bzycj-2018-0381.YU J L, YAO F T, YU X Z, et al. Experimental study on the influence of high temperature and high pressure on the upper limit of explosion of ethane in oxygen [J]. Explosion and Shock Waves, 2019, 39(12): 122101. DOI: 10.11883/bzycj-2018-0381. [21] 张永刚, 杜志国, 张利军, 等. 乙烷丙烷裂解研究 [J]. 乙烯工业, 2018, 30(2): 6–7. DOI: 10.3969/j.issn.1671-7120.2018.02.002.ZHANG Y G, DU Z G, ZHANG L J, et al. Study on pyrolysis of ethane and propane [J]. Ethylene Industry, 2018, 30(2): 6–7. DOI: 10.3969/j.issn.1671-7120.2018.02.002. [22] Determination of the explosion limits and the limiting oxygen concentration (LOC) for flammable gases and vapours: BS EN 1839—2017 [S]. Brussels: European Committee for Standardization, 2017. [23] 空气中可燃气体爆炸极限测定方法: GB/T 12474—2008 [S]. 天津: 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会, 2008. [24] 李法社, 王华. 高等燃烧学 [M]. 北京: 科学出版社, 2016: 68. [25] 沈晓波. 密闭空间内典型可燃气体层流预混火焰传播动力学及其化学反应机理研究 [D]. 合肥: 中国科学技术大学, 2014: 73–74.SHEN X B. Study of laminar premixed flame propagating in confined spaces and chemical kinetic mechanisms for typical combustible gases [D]. Heifei, Anhui, China: University of Science and Technology of China, 2014: 73–74. [26] BYCHKOV V V, LIBERMAN M A. Dynamics and stability of premixed flames [J]. Physics Reports-Review Section of Physics Letters, 2000, 325(4/5): 115–237. DOI: 10.1016/s0370-1573(99)00081-2. [27] WANG T, LUO Z M, WEN H, et al. The explosion enhancement of methane-air mixtures by ethylene in a confined chamber [J]. Energy, 2021, 214: 119042. DOI: 10.1016/j.energy.2020.119042. [28] DAVIS S G, LAW C K. Determination of and fuel structure effects on laminar flame speeds of C-1 to C-8 hydrocarbons [J]. Combustion Science and Technology, 1998, 140(1): 427–449. DOI: 10.1080/00102209808915781. [29] KONDO S, TAKIZAWA K, TAKAHASHI A, et al. Extended Le Chatelier’s formula for carbon dioxide dilution effect on flammability limits [J]. Journal of Hazardous Materials, 2006, 138(1): 1–8. DOI: 10.1016/j.jhazmat.2006.05.035. -






 下载:
下载: