Role mechanism of the postsynaptic scaffold protein Preso in the induction of post-traumatic stress disorder by blast traumatic brain injury
-
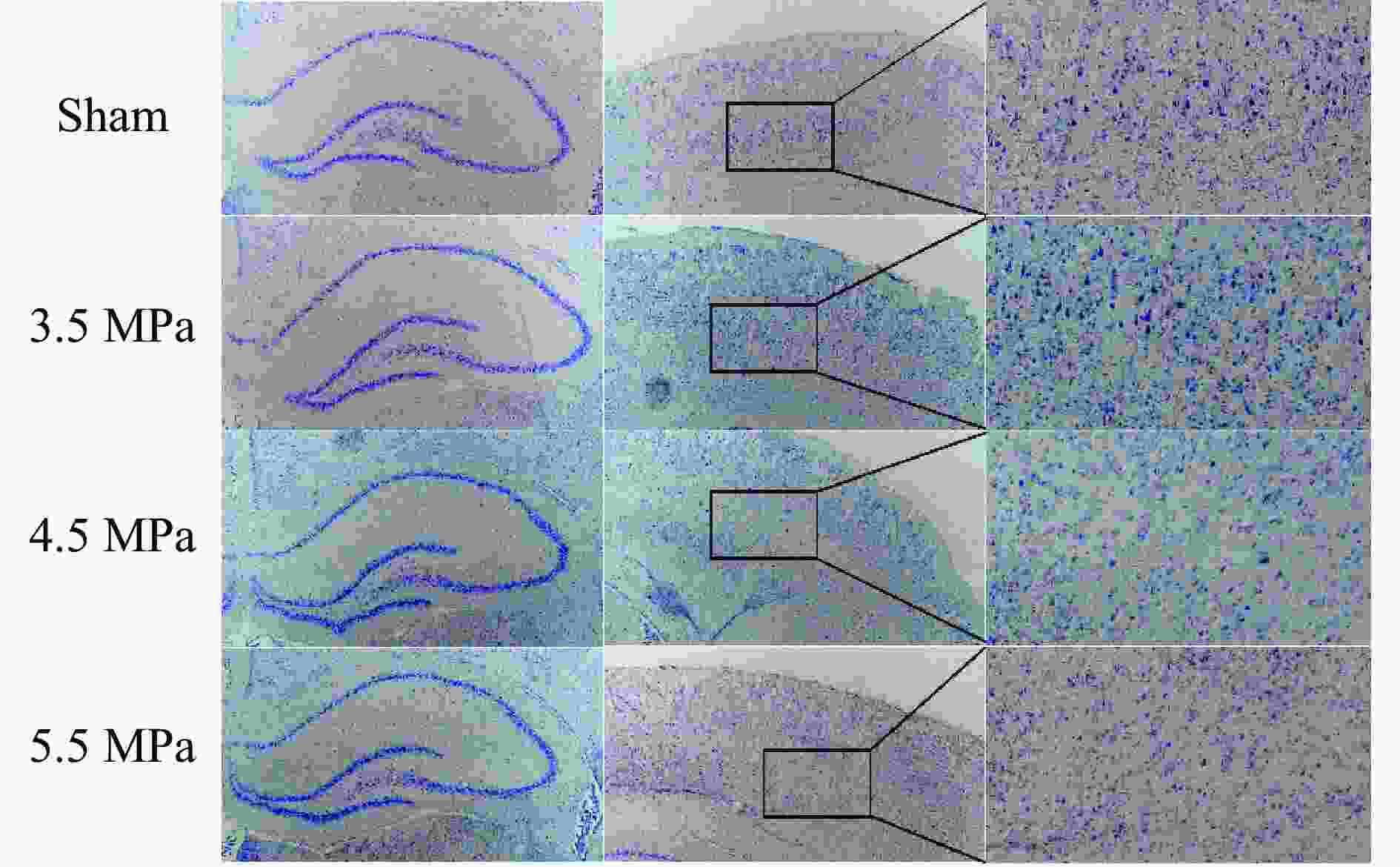
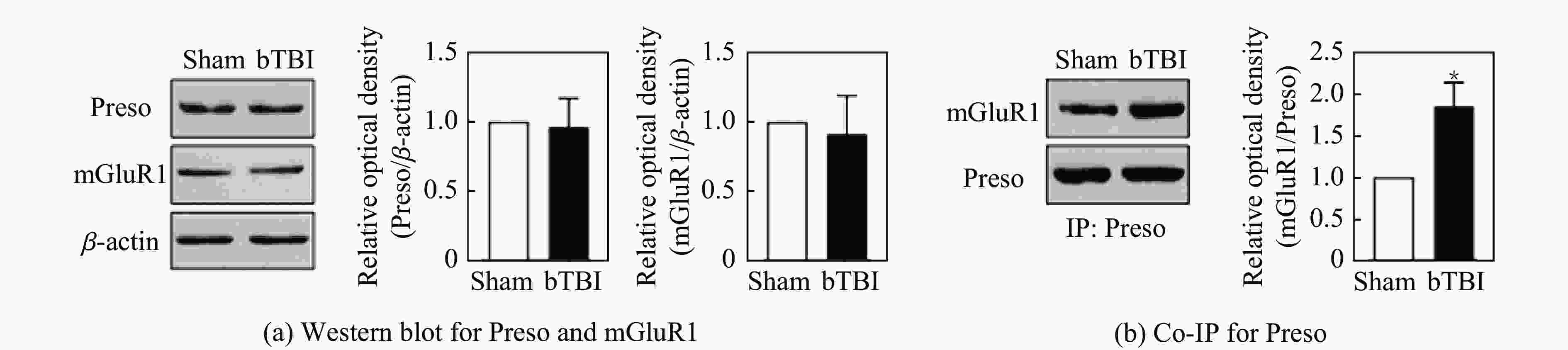
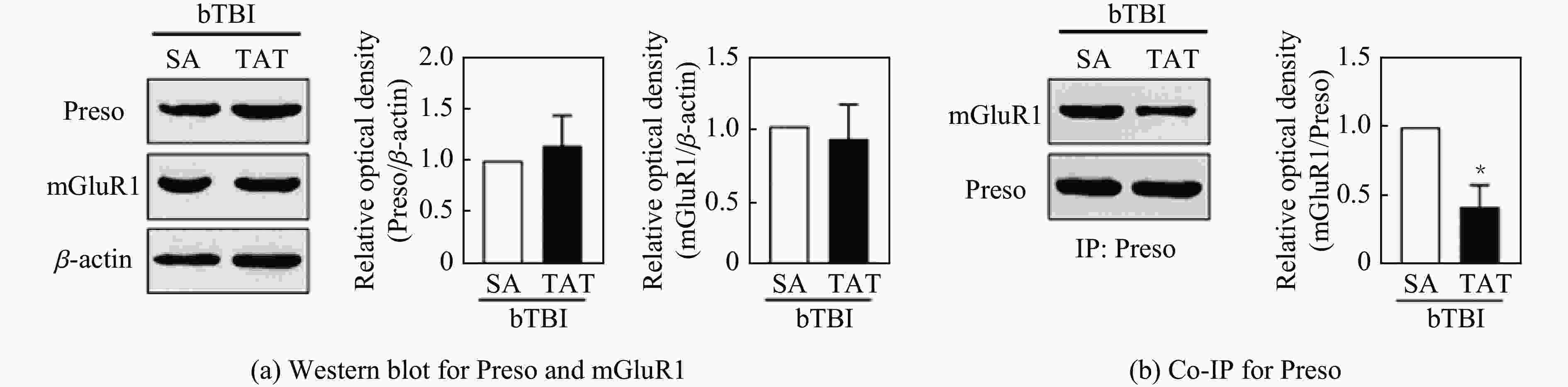
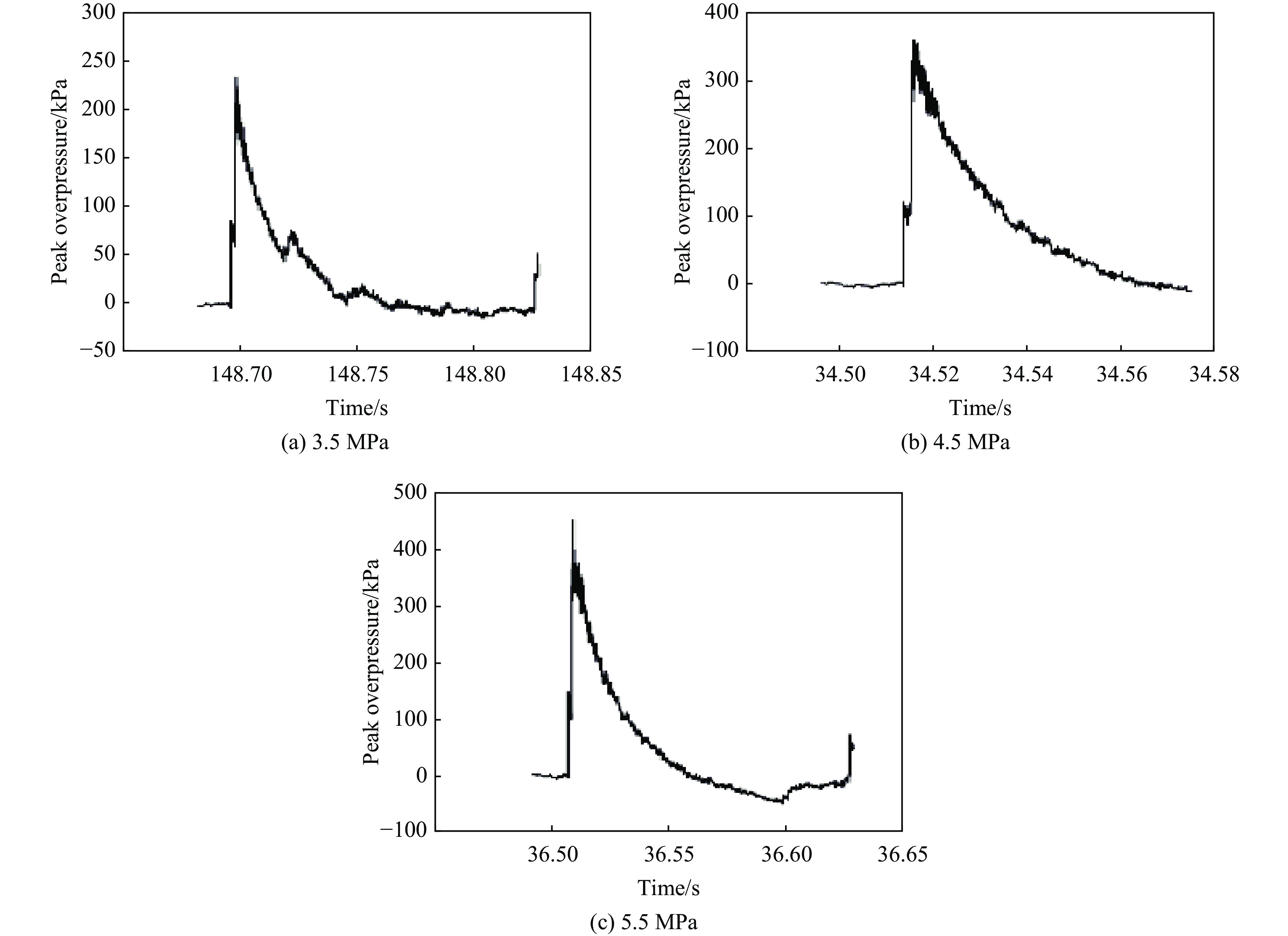
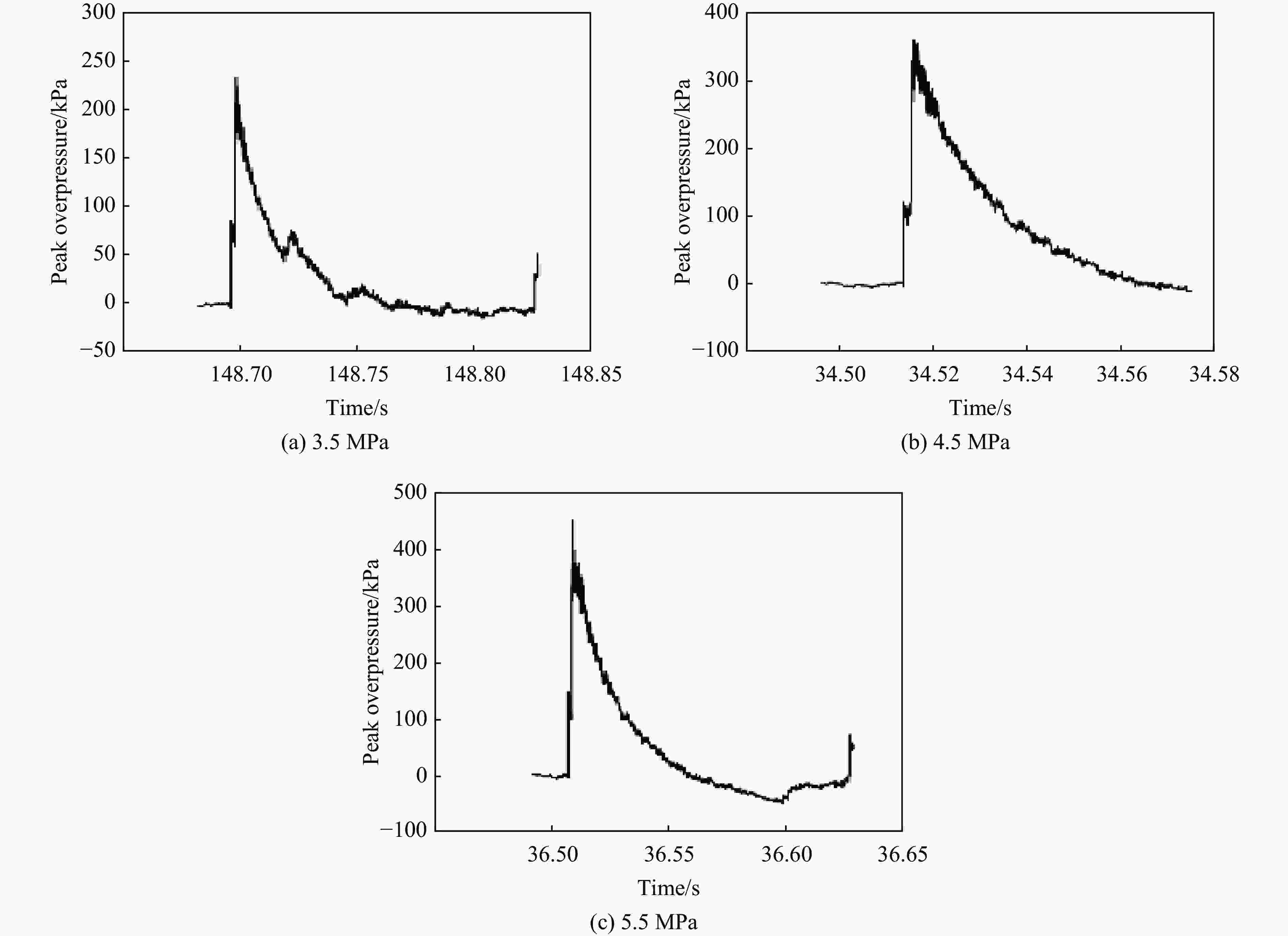
摘要: 将36只雄性C57小鼠随机分为对照组(Sham组)、3.5 MPa 颅脑冲击伤(blast-related traumatic brain injury, bTBI)组、4.5 MPa bTBI组、5.5 MPa bTBI组、4.5 MPa bTBI+生理盐水组(bTBI+SA组)、4.5 MPa bTBI+小分子多肽组(bTBI+TAT-FERM组),每组6只;将12只 Preso -/-小鼠随机分为Sham组和 4.5 MPa bTBI组,每组6只。对小鼠进行bTBI造模,完成后常规饲养2周,4.5 MPa bTBI+生理盐水组和4.5 MPa bTBI+TAT-FERM组在bTBI造模后每天通过尾静脉给药1次,连续给药5 d。与对照组相比,3.5 MPa bTBI组小鼠焦虑抑郁行为改变不显著;4.5 MPa bTBI和5.5 MPa bTBI组小鼠出现创伤后应激障碍(posttraumatic stress disorder, PTSD)样症状。与对照组相比,4.5 MPa bTBI组Preso/mGluR1复合体形成增加,使用TAT-FERM可阻断Preso与mGluR1的相互作用,可在不改变Preso/mGluR1复合体组成分子蛋白表达的情况下抑制Preso/mGluR1复合体形成,并且改善bTBI所导致的PTSD症状。bTBI促进Preso/mGluR1复合体形成是bTBI诱致PTSD症状的重要分子病理机制,通过阻断Preso与mGluR1相互作用可减轻bTBI对PTSD的影响,进而为治疗bTBI相关的PTSD提供了潜在靶点。Abstract: To investigate the mechanism of post-synaptic scaffold protein Preso in the exacerbation of post-traumatic stress disorder (PTSD) by blast-related traumatic brain injury (bTBI), thirty-six male C57 mice were randomly divided into the control group (Sham group), 3.5 MPa bTBI group, 4.5 MPa bTBI group, 5.5 MPa bTBI group, 4.5 MPa bTBI+saline group, 4.5 MPa bTBI+small molecule interfering peptide (TAT-FERM) group, and 6 mice in each group. And twelve Preso-/- mice were randomly divided into sham group and 4.5 MPa bTBI group, with 6 mice in each group. The mice were subjected to bTBI modelling and were routinely kept for 2 weeks after completion. 4.5 MPa bTBI+saline group and 4.5 MPa bTBI+TAT-FERM group were administered once a day through the tail vein for 5 consecutive days after bTBI modelling. Compared with the control group, the anxiety and depression behavior of 3.5 MPa bTBI mice was not significantly changed. Mice in the 4.5 MPa bTBI and 5.5 MPa bTBI groups showed significant PTSD symptoms and promoted the formation of the Preso/mGluR1 complex. The use of TAT-FERM blocked the interaction between Preso and mGluR1, inhibited the formation of Preso/mGluR1 complex without altering the expression of Preso/mGluR1 complex component proteins, and ameliorated PTSD symptoms caused by bTBI. Results display that the promotion of Preso/mGluR1 complex formation by bTBI is an important molecular pathological mechanism by which bTBI induces PTSD symptoms. The effect of bTBI on PTSD can be attenuated by blocking the interaction between Preso and mGluR1, providing a potential target for the treatment of bTBI-associated PTSD.
-
表 1 4组小鼠旷场实验结果对比
Table 1. Comparison of results of four groups of mice in open-field experiment
分组 进入中心区域次数 中心区域运动距离百分比/% Sham 5.83±0.65 15.12±1.74 3.5 MPa bTBI 5.67±0.52 14.75±1.55 4.5 MPa bTBI 4.33±0.44 10.43±1.16 5.5 MPa bTBI 3.50±0.55 7.47±0.37 表 2 4组小鼠高架十字迷宫实验结果的比较
Table 2. Comparison of results of elevated cross maze experiments in four groups of mice
分组 $ {\mathrm{\gamma }}_{{n}_{\mathrm{o}\mathrm{e}}} $/% $ {\mathrm{\gamma }}_{{n}_{\mathrm{o}\mathrm{t}}} $/% Sham 14.02±1.32 12.15±1.55 3.5 MPa bTBI 10.57±0.83 10.03±0.54 4.5 MPa bTBI 9.41±0.96 8.11±0.88 5.5 MPa bTBI 5.38±0.75 4.95±1.28 表 3 2组Preso-/-小鼠旷场实验结果对比
Table 3. Comparison of results of two groups of Preso-/- mice in open-field experiment
分组 进入中心区域次数 中心区域运动距离百分比/% Sham 6.33±0.65 14.81±0.84 bTBI 5.33±0.52 13.94±1.35 表 4 2组Preso-/-小鼠高架十字迷宫实验结果比较
Table 4. Comparison of results of elevated cross maze experiments in two groups of Preso-/- mice
分组 $ {\mathrm{\gamma }}_{{n}_{\mathrm{o}\mathrm{e}}} $/% $ {\mathrm{\gamma }}_{{n}_{\mathrm{o}\mathrm{t}}} $/% Sham 13.34±0.98 11.15±1.43 bTBI 12.55±1.28 10.03±1.24 表 5 2组bTBI小鼠旷场实验结果对比
Table 5. Comparison of results of two groups of bTBI mice in open-field experiment
给药分组 进入中心区域次数 中心区域运动距离百分比/% SA 3.67±0.75 8.08±0.86 TAT-FERM 4.50±0.89 12.44±0.45 表 6 2组bTBI小鼠高架十字迷宫实验结果比较
Table 6. Comparison of results of elevated cross maze experiments in two groups of bTBI mice
分组 $ {\mathrm{\gamma }}_{{n}_{\mathrm{o}\mathrm{e}}} $/% $ {\mathrm{\gamma }}_{{n}_{\mathrm{o}\mathrm{t}}} $/% SA 5.21±0.76 5.18±0.45 TAT-FERM 9.79±1.15 9.32±0.59 -
[1] LINDBERG M A, MOY MARTIN E M, MARION D W. Military traumatic brain injury: the history, impact, and future [J]. Journal of Neurotrauma, 2022, 39(17/18): 1133–1145. DOI: 10.1089/neu.2022.0103. [2] TROYANSKAYA M, PASTOREK N J, SCHEIBEL R S, et al. Combat exposure, PTSD symptoms, and cognition following blast-related traumatic brain injury in OEF/OIF/OND service members and veterans [J]. Military Medicine, 2015, 180(3): 285–289. DOI: 10.7205/MILMED-D-14-00256. [3] KAPLAN G B, LEITE-MORRIS K A, WANG L, et al. Pathophysiological bases of comorbidity: traumatic brain injury and post-traumatic stress disorder [J]. Journal of Neurotrauma, 2018, 35(2): 210–225. DOI: 10.1089/neu.2016.4953. [4] JAMJOOM A A B, RHODES J, ANDREWS P J D, et al. The synapse in traumatic brain injury [J]. Brain, 2021, 144(1): 18–31. DOI: 10.1093/brain/awaa321. [5] HU J H, YANG L L, KAMMERMEIER P J, et al. Preso1 dynamically regulates group I metabotropic glutamate receptors [J]. Nature Neuroscience, 2012, 15(6): 836–844. DOI: 10.1038/nn.3103. [6] ZHANG Z Y, GAO X Y, TIAN Z C, et al. Preso enhances mGluR1-mediated excitotoxicity by modulating the phosphorylation of mGluR1-Homer1 complex and facilitating an ER stress after traumatic brain injury [J]. Cell Death Discovery, 2024, 10(1): 153. DOI: 10.1038/s41420-024-01916-5. [7] RACE N S, ANDREWS K D, LUNGWITZ E A, et al. Psychosocial impairment following mild blast-induced traumatic brain injury in rats [J]. Behavioural Brain Research, 2021, 412: 113405. DOI: 10.1016/j.bbr.2021.113405. [8] KIM S Y, YEH P H, OLLINGER J M, et al. Military-related mild traumatic brain injury: clinical characteristics, advanced neuroimaging, and molecular mechanisms [J]. Translational Psychiatry, 2023, 13(1): 289. DOI: 10.1038/s41398-023-02569-1. [9] LAI C, KOSTAS-POLSTON E A, ENGLER M B, et al. Prevalence of PTSD in active duty members with mild traumatic brain injury: systematic review and meta-analysis [J]. Military Medicine, 2024, 189(7/8): e1454–e1461. DOI: 10.1093/milmed/usae272. [10] CHEN T, ZHU J, WANG Y H, et al. Arc silence aggravates traumatic neuronal injury via mGluR1-mediated ER stress and necroptosis [J]. Cell Death and Disease, 2020, 11(1): 4. DOI: 10.1038/s41419-019-2198-5. [11] HENTER I D, PARK L T, ZARATE C A. Novel glutamatergic modulators for the treatment of mood disorders: current status [J].CNS Drugs, 2021, 35(5): 527–543. DOI: 10.1007/s40263-021-00816-x. [12] BARACALDO-SANTAMARÍA D, ARIZA-SALAMANCA D F, CORRALES-HERNÁNDEZ M G, et al. Revisiting excitotoxicity in traumatic brain injury: from bench to bedside [J]. Pharmaceutics, 2022, 14(1): 152. DOI: 10.3390/pharmaceutics14010152. [13] LEE H W, CHOI J, SHIN H, et al. Preso, a novel PSD-95-interacting FERM and PDZ domain protein that regulates dendritic spine morphogenesis [J]. Journal of Neuroscience, 2008, 28(53): 14546–14556. DOI: 10.1523/JNEUROSCI.3112-08.2008. -






 下载:
下载: