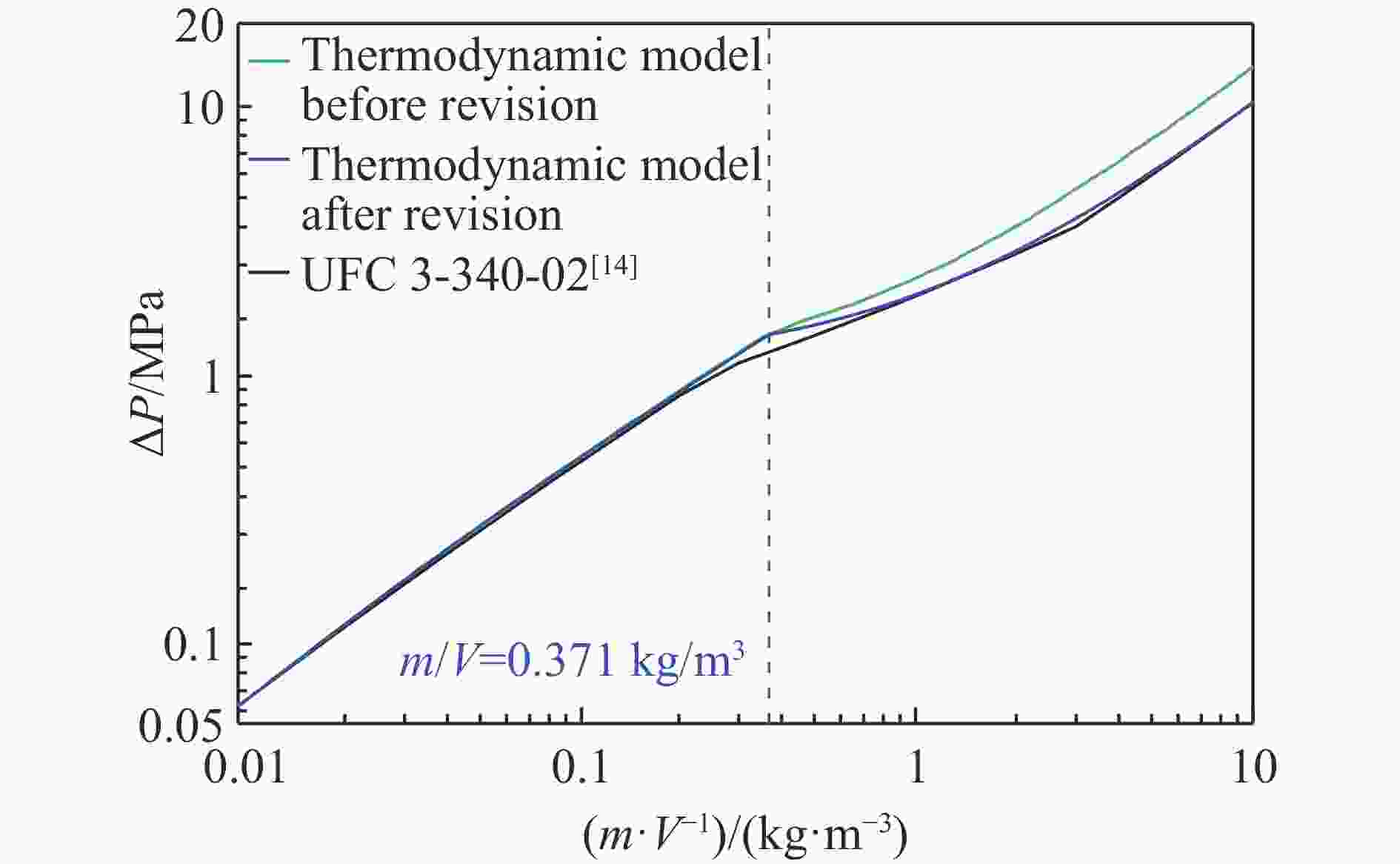
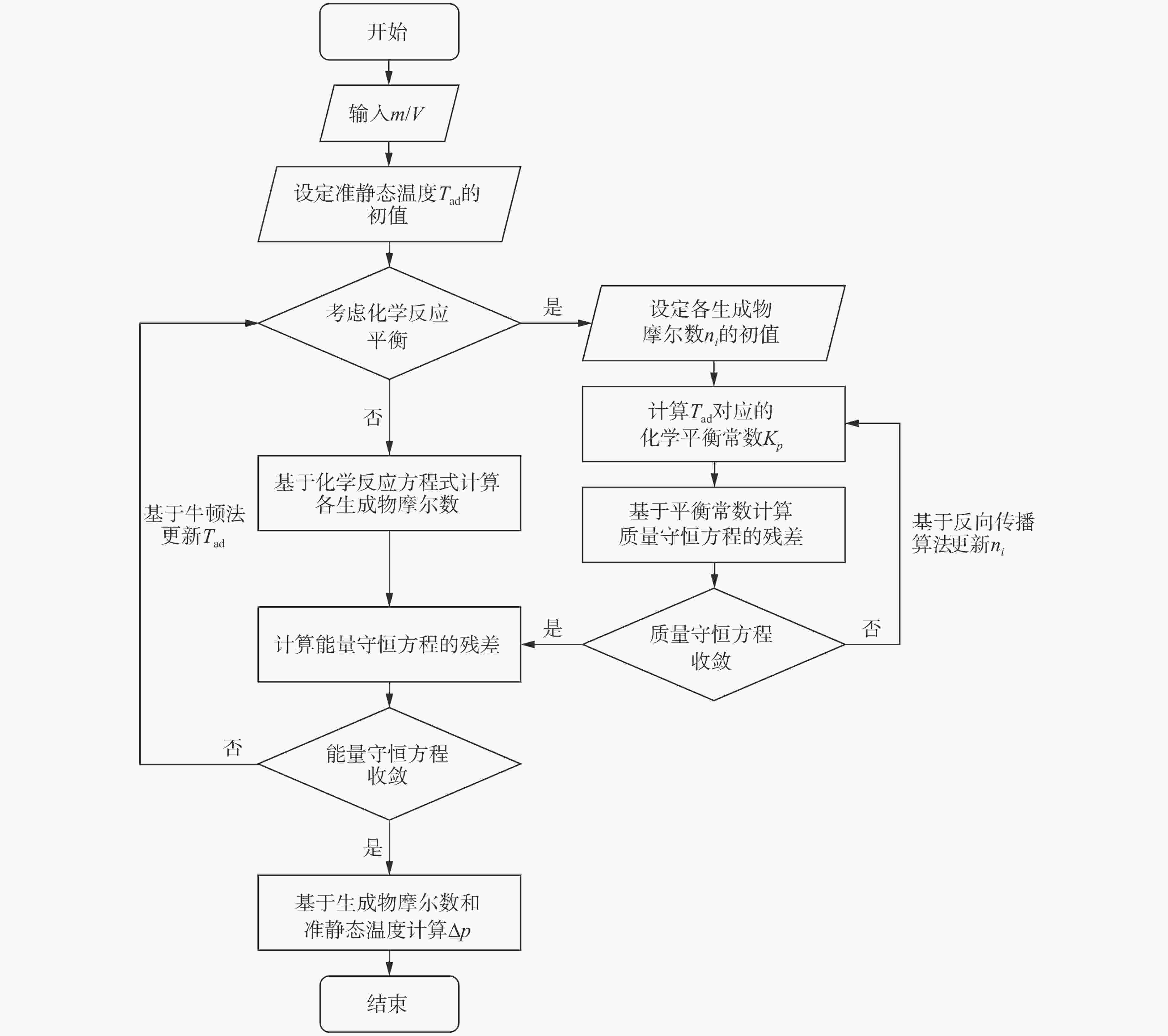
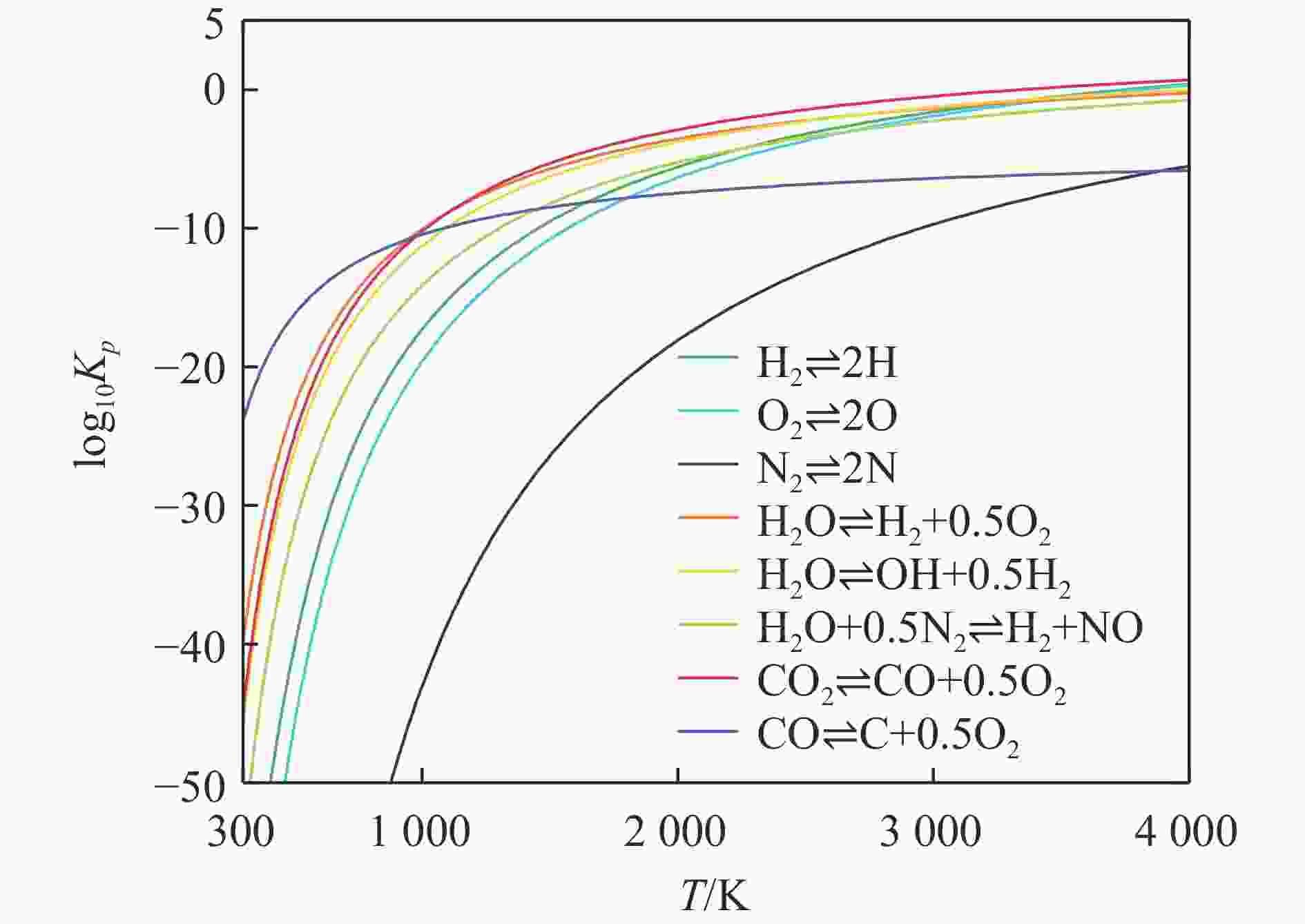
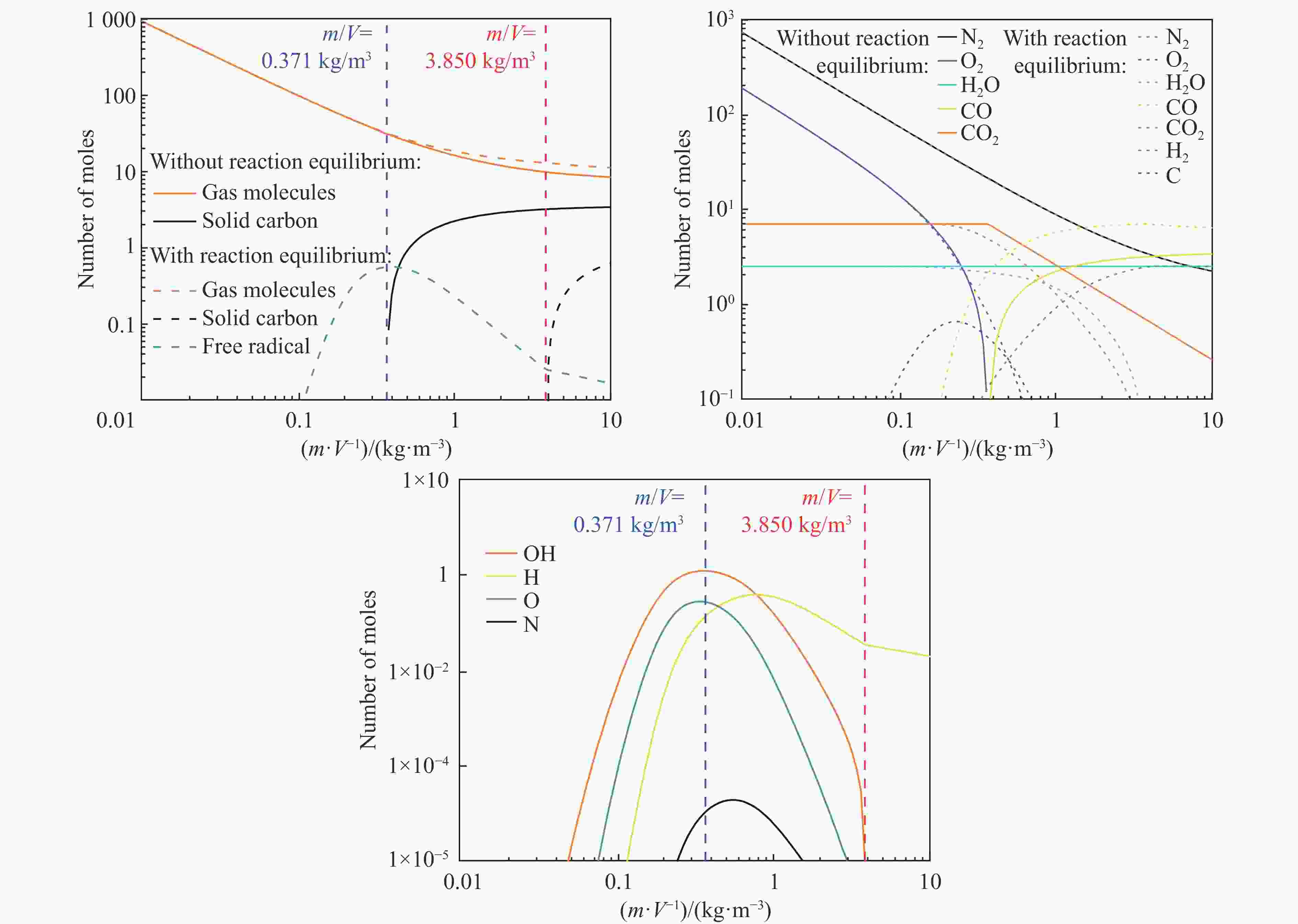
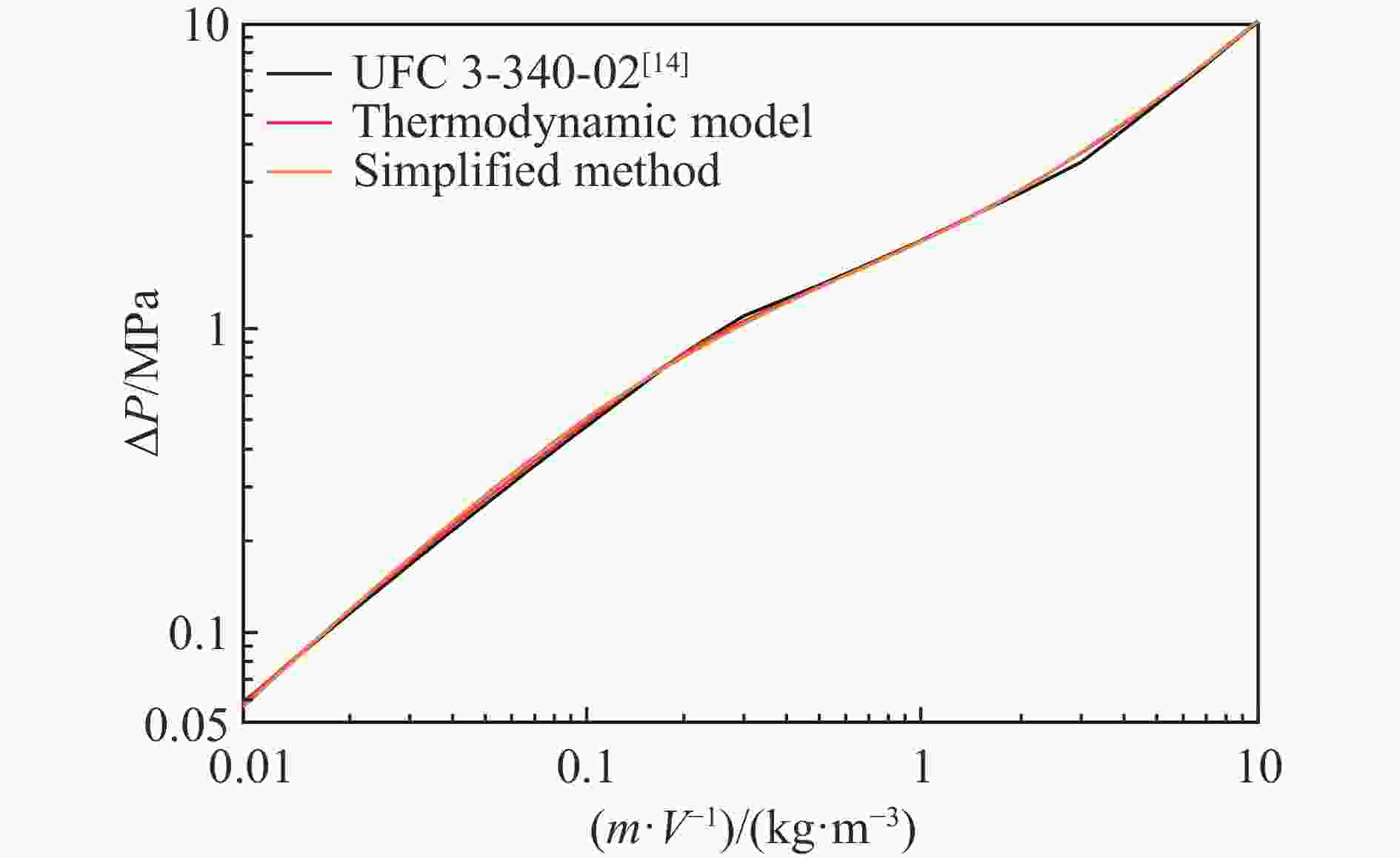
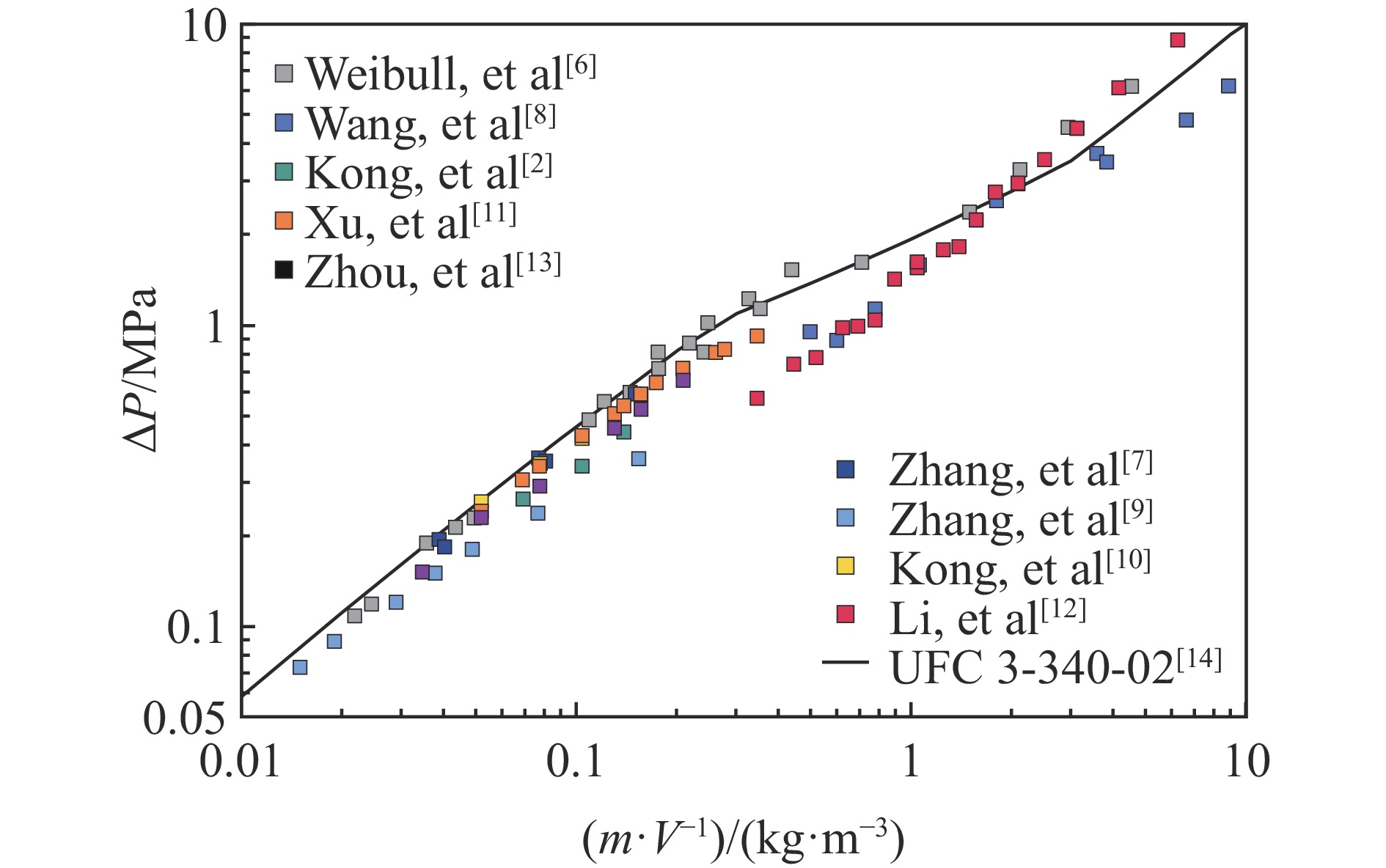
| [1] |
EDRI I, FELDGUN V R, KARINSKI Y S, et al. On blast pressure analysis due to a partially confined explosion: Ⅲ. Afterburning effect [J]. International Journal of Protective Structures, 2012, 3(3): 311–331. DOI: 10.1260/2041-4196.3.3.311.
|
| [2] |
KONG X S, ZHOU H, ZHENG C, et al. An experimental study on the mitigation effects of fine water mist on confined-blast loading and dynamic response of steel plates [J]. International Journal of Impact Engineering, 2019, 134: 103370. DOI: 10.1016/j.ijimpeng.2019.103370.
|
| [3] |
李营, 杜志鹏, 陈赶超, 等. 舰艇爆炸毁伤与防护若干关键问题研究进展 [J]. 中国舰船研究, 2024, 19(3): 3–60. DOI: 10.19693/j.issn.1673-3185.03930.Li Y, DU Z P, CHEN G C, et al. Fundamental problems in blast-induced damage and protection of naval vessels: a state-of-the-art review [J]. Chinese Journal of Ship Research, 2024, 19(3): 3–60. DOI: 10.19693/j.issn.1673-3185.03930.
|
| [4] |
孔祥韶, 王子棠, 况正, 等. 密闭空间内爆炸载荷抑制效应实验研究 [J]. 爆炸与冲击, 2021, 41(6): 062901. DOI: 10.11883/bzycj-2020-0193.KONG X S, WANG Z T, KUANG Z, et al. Experimental study on the mitigation effects of confined-blast loading [J]. Explosion and Shock Waves, 2021, 41(6): 062901. DOI: 10.11883/bzycj-2020-0193.
|
| [5] |
GRISARO H Y. Pressure-Impulse diagrams for assessment of structural response due to a fully confined explosion [J]. Engineering Failure Analysis, 2023, 146: 107103. DOI: 10.1016/j.engfailanal.2023.107103.
|
| [6] |
WEIBULL H R W. Pressures recorded in partially closed chambers at explosion of TNT charges [J]. Annals of the New York Academy of Sciences, 1968, 152(1): 357–361. DOI: 10.1111/j.1749-6632.1968.tb11987.x.
|
| [7] |
ZHANG F, ANDERSON J, YOSHINAKA A. Post-detonation energy release from TNT-aluminum explosives [J]. AIP Conference Proceedings, 2007, 955(1): 885–888. DOI: 10.1063/1.2833268.
|
| [8] |
王等旺, 张德志, 李焰, 等. 爆炸容器内准静态气压实验研究 [J]. 兵工学报, 2012, 33(12): 1493–1497. DOI: 10.3969/j.issn.1000-1093.2012.12.014.WANG D W, ZHANG D Z, LI Y, et al. Experiment investigation on quasi-static pressure in explosion containment vessels [J]. Acta Armamentarii, 2012, 33(12): 1493–1497. DOI: 10.3969/j.issn.1000-1093.2012.12.014.
|
| [9] |
张玉磊, 苏健军, 李芝绒, 等. TNT内爆炸准静态压力特性 [J]. 爆炸与冲击, 2018, 38(6): 1429–1434. DOI: 10.11883/bzycj-2017-0170.ZHANG Y L, SU J J, LI Z R, et al. Quasi-static pressure characteristic of TNT’s internal explosion [J]. Explosion and Shock Waves, 2018, 38(6): 1429–1434. DOI: 10.11883/bzycj-2017-0170.
|
| [10] |
孔祥韶, 况正, 郑成, 等. 舱室密闭空间中爆炸载荷燃烧增强效应试验研究 [J]. 兵工学报, 2020, 41(1): 75–85. DOI: 10.3969/j.issn.1000-1093.2020.01.009.KONG X S, KUANG Z, ZHENG C, et al. Experimental study of afterburning enhancement effect for blast load in confined compartment space [J]. Acta Armamentarii, 2020, 41(1): 75–85. DOI: 10.3969/j.issn.1000-1093.2020.01.009.
|
| [11] |
徐敬博. 舱室内爆载荷作用下结构动态响应相似规律研究 [D]. 武汉: 武汉理工大学, 2019: 32–69. DOI: 10.27381/d.cnki.gwlgu.2019.001805.XU J B. Study on similarity laws of dynamic response for structures under blast load in cabin [D]. Wuhan: Wuhan University of Technology, 2019: 32–69. DOI: 10.27381/d.cnki.gwlgu.2019.001805.
|
| [12] |
李营, 张磊, 杜志鹏, 等. 舱内爆炸准静态压力形成机理的研究 [J]. 中国造船, 2020, 61(2): 28–34. DOI: 10.3969/j.issn.1000-4882.2020.02.003.LI Y, ZHANG L, DU Z P, et al. Theoretical and experimental study on formation of quasi-static pressure in internal blast [J]. Shipbuilding of China, 2020, 61(2): 28–34. DOI: 10.3969/j.issn.1000-4882.2020.02.003.
|
| [13] |
ZHOU H, ZHENG C, YUE X S, et al. TNT equivalency method in confined space based on steel plate deformation [J]. International Journal of Impact Engineering, 2023, 178: 104587. DOI: 10.1016/j.ijimpeng.2023.104587.
|
| [14] |
US Department of Defense. UFC 3-340-02 Structures to resist the effects of accidental explosions [S]. Washington: UFC, 2008.
|
| [15] |
TYAS A, REAY J J, FAY S D, et al. Experimental studies of the effect of rapid afterburn on shock development of near-field explosions [J]. International Journal of Protective Structures, 2016, 7(3): 452–465. DOI: 10.1177/2041419616665931.
|
| [16] |
FEDINA E, FUREBY C. Investigating ground effects on mixing and afterburning during a TNT explosion [J]. Shock Waves, 2013, 23(3): 251–261. DOI: 10.1007/s00193-012-0420-9.
|
| [17] |
ZHOU H, YUE X S, ZHENG C, et al. Dynamic behavior of steel plates subjected to confined blast loading considering afterburning effect [J]. International Journal of Impact Engineering, 2024, 188: 104934. DOI: 10.1016/j.ijimpeng.2024.104934.
|
| [18] |
KIM C K, MOON J G, LAI M C, et al. Afterburning of TNT explosive products in air with aluminum particles [C]//Proceedings of the 46th AIAA Aerospace Sciences Meeting and Exhibit. Reno: AIAA, 2008: 1029–1039. DOI: 10.2514/6.2008-1029.
|
| [19] |
FARRIMOND D G, WOOLFORD S, BARR A D, et al. Experimental studies of confined detonations of plasticized high explosives in inert and reactive atmospheres [J]. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, 2024, 480(2294): 20240061. DOI: 10.1098/rspa.2024.0061.
|
| [20] |
CARLSON R W. Confinement of an explosion by a steel vessel: LA-390 [R]. Los Alamos: LANL, 1945.
|
| [21] |
刘文祥, 张德志, 钟方平, 等. 球形爆炸容器内炸药爆炸形成的准静态气体压力 [J]. 爆炸与冲击, 2018, 38(5): 1045–1050. DOI: 10.11883/bzycj-2017-0056.LIU W X, ZHANG D Z, ZHONG F P, et al. Quasi-static gas pressure generated by explosive charge blasting in a spherical explosion containment vessel [J]. Explosion and Shock Waves, 2018, 38(5): 1045–1050. DOI: 10.11883/bzycj-2017-0056.
|
| [22] |
FELDGUN V R, KARINSKI Y S, EDRI I, et al. Prediction of the quasi-static pressure in confined and partially confined explosions and its application to blast response simulation of flexible structures [J]. International Journal of Impact Engineering, 2016, 90: 46–60. DOI: 10.1016/j.ijimpeng.2015.12.001.
|
| [23] |
KINNEY G F, SEWELL R G S, GRAHAM K J. Peak overpressures for internal blast: NWC TP 6089 [R]. California: Naval Weapons Center, 1979.
|
| [24] |
钟巍, 田宙, 赵阳. 考虑约束爆炸后产物发生化学反应的约束空间内准静态温度计算 [J]. 爆炸与冲击, 2015, 35(6): 777–784. DOI: 10.11883/1001-1455(2015)06-0777-08.ZHONG W, TIAN Z, ZHAO Y. Calculation of the quasi-static temperature of confined explosions in consideration of the effect of the chemical reactions with detonation products [J]. Explosion and Shock Waves, 2015, 35(6): 777–784. DOI: 10.11883/1001-1455(2015)06-0777-08.
|
| [25] |
徐维铮, 吴卫国. 密闭空间内爆炸准静态压力理论计算研究 [J]. 中国舰船研究, 2019, 14(5): 124–130. DOI: 10.19693/j.issn.1673-3185.01368.XU W Z, WU W G. Study on theoretical calculation of quasi-static pressure for explosion in confined space [J]. Chinese Journal of Ship Research, 2019, 14(5): 124–130. DOI: 10.19693/j.issn.1673-3185.01368.
|
| [26] |
EDRI I E, GRISARO H Y, YANKELEVSKY D Z. TNT equivalency in an internal explosion event [J]. Journal of Hazardous Materials, 2019, 374: 248–257. DOI: 10.1016/j.jhazmat.2019.04.043.
|
| [27] |
岳学森, 周沪, 孔祥韶, 等. 舱室内爆载荷燃烧增强效应试验及仿真研究 [J]. 中国舰船研究, 2023, 18(4): 223–232. DOI: 10.19693/j.issn.1673-3185.02708.YUE X S, ZHOU H, KONG X S, et al. Experimental and simulation study of afterburning effect for blast load in confined cabin [J]. Chinese Journal of Ship Research, 2023, 18(4): 223–232. DOI: 10.19693/j.issn.1673-3185.02708.
|
| [28] |
岳学森. 舰船舱内爆炸载荷燃烧增强效应及抑制方法研究 [D]. 武汉: 武汉理工大学, 2022. DOI: 10.27381/d.cnki.gwlgu.2022.001554.YUE X S. Study on afterburning effect and mitigation method of blast load in confined cabin [D]. Wuhan: Wuhan University of Technology, 2022. DOI: 10.27381/d.cnki.gwlgu.2022.001554.
|
| [29] |
Л. П. 奥尔连科. 爆炸物理学(上册) [M]. 3版. 孙承纬, 译. 北京: 科学出版社, 2011: 286–293.ОРЛЕНКО Л П. Explosion physics [M]. 3rd ed. SUN C W, trans. Beijing: Science Press, 2011: 286–293.
|
| [30] |
MANION J A. NIST chemical kinetics database [DB/OL]. [2024-10-14]. https://webbook.nist.gov/chemistry/name-ser/.
|
| [31] |
BUNDY F P, BASSETT W A, WEATHERS M S, et al. The pressure-temperature phase and transformation diagram for carbon; updated through 1994 [J]. Carbon, 1996, 34(2): 141–153. DOI: 10.1016/0008-6223(96)00170-4.
|
| [32] |
ORNELLAS D L. Calorimetric detemination of the heat and products of detonation for explosives: October 1961 to April 1982: UCRL-52821 [R]. California: Berkeley Lawrence Livermore Laboratory, 1982.
|
| [33] |
王中友, 李星翰, 甘云丹, 等. 爆热弹中产物组分演化的计算研究 [J]. 火炸药学报, 2022, 45(2): 229–242. DOI: 10.14077/j.issn.1007-7812.202112008.WANG Z Y, LI X H, GAN Y D, et al. Study on thermodynamic evolution of detonation products in the detonation bomb test [J]. Chinese Journal of Explosives & Propellants, 2022, 45(2): 229–242. DOI: 10.14077/j.issn.1007-7812.202112008.
|
| [34] |
严传俊, 范玮. 燃烧学 [M]. 2版. 西安: 西北工业大学出版社, 2005.
|
| [35] |
ORNELLAS D L. The heat and products of detonation in a calorimeter of CNO, HNO, CHNF, CHNO, CHNOF, and CHNOSi explosives [J]. Combustion and Flame, 1974, 23(1): 37–46. DOI: 10.1016/S0010-2180(74)80025-8.
|






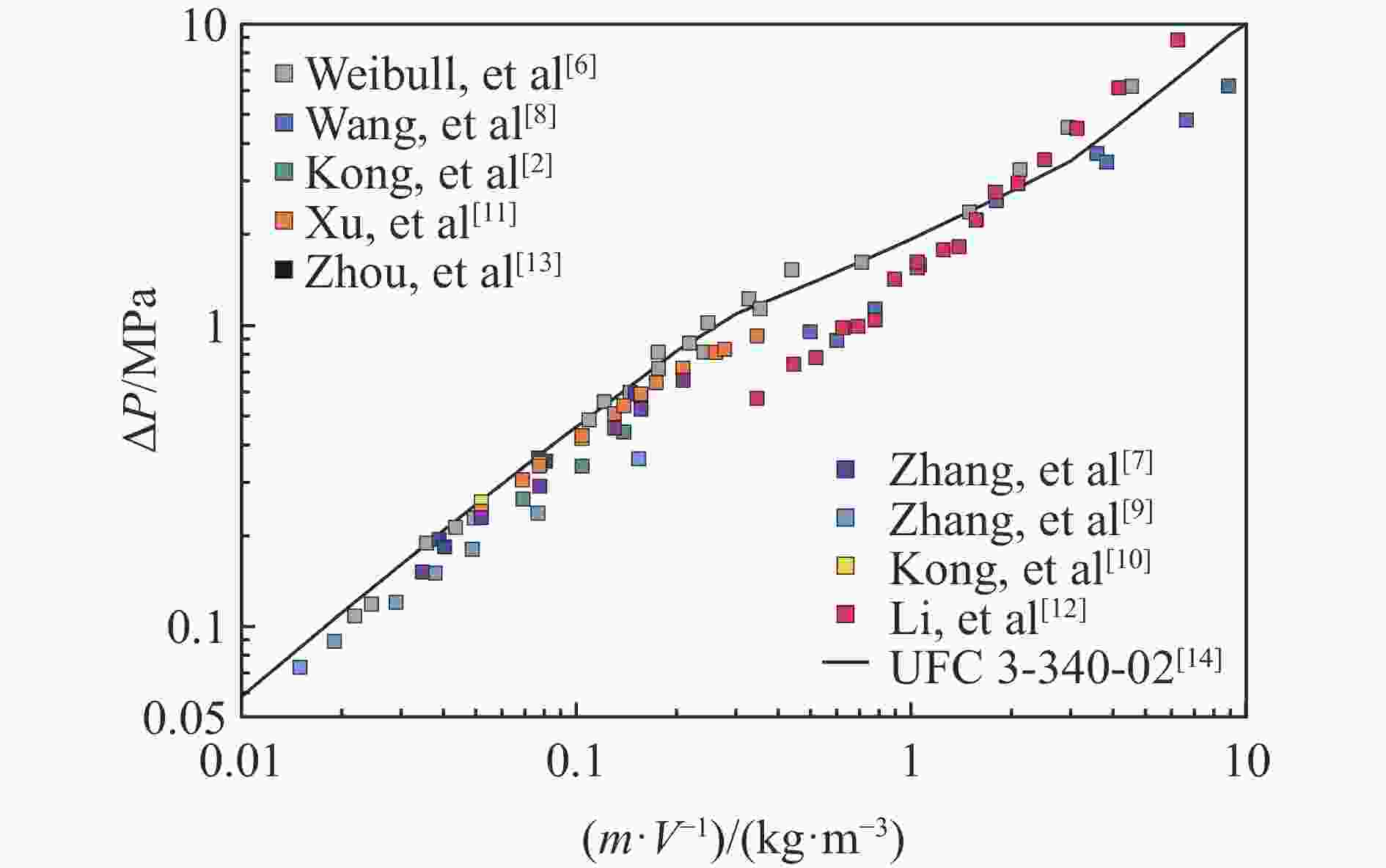
 下载:
下载: