Energy dynamics and power evaluation method of high pressure hydrogen storage tank explosion
-
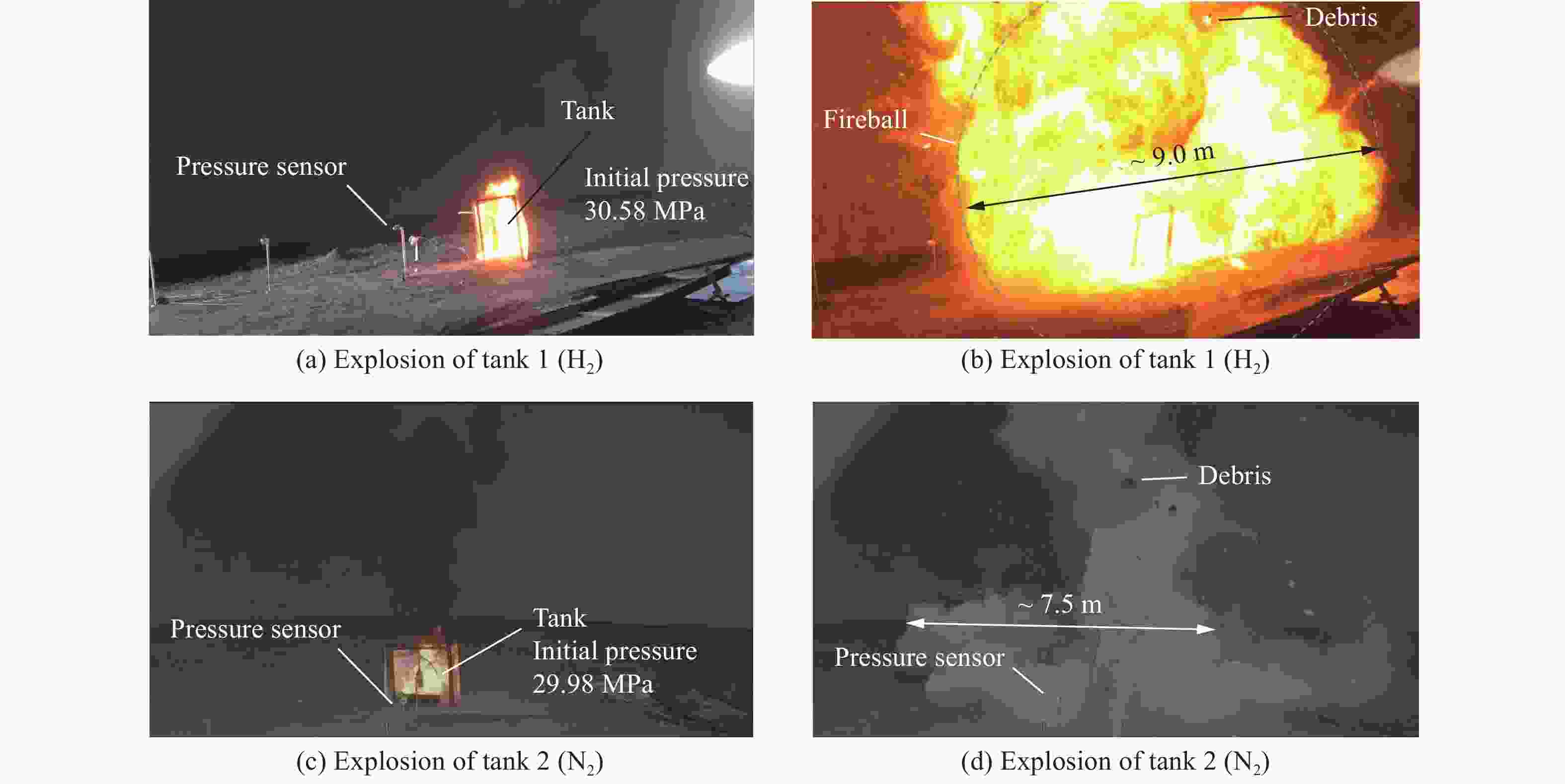
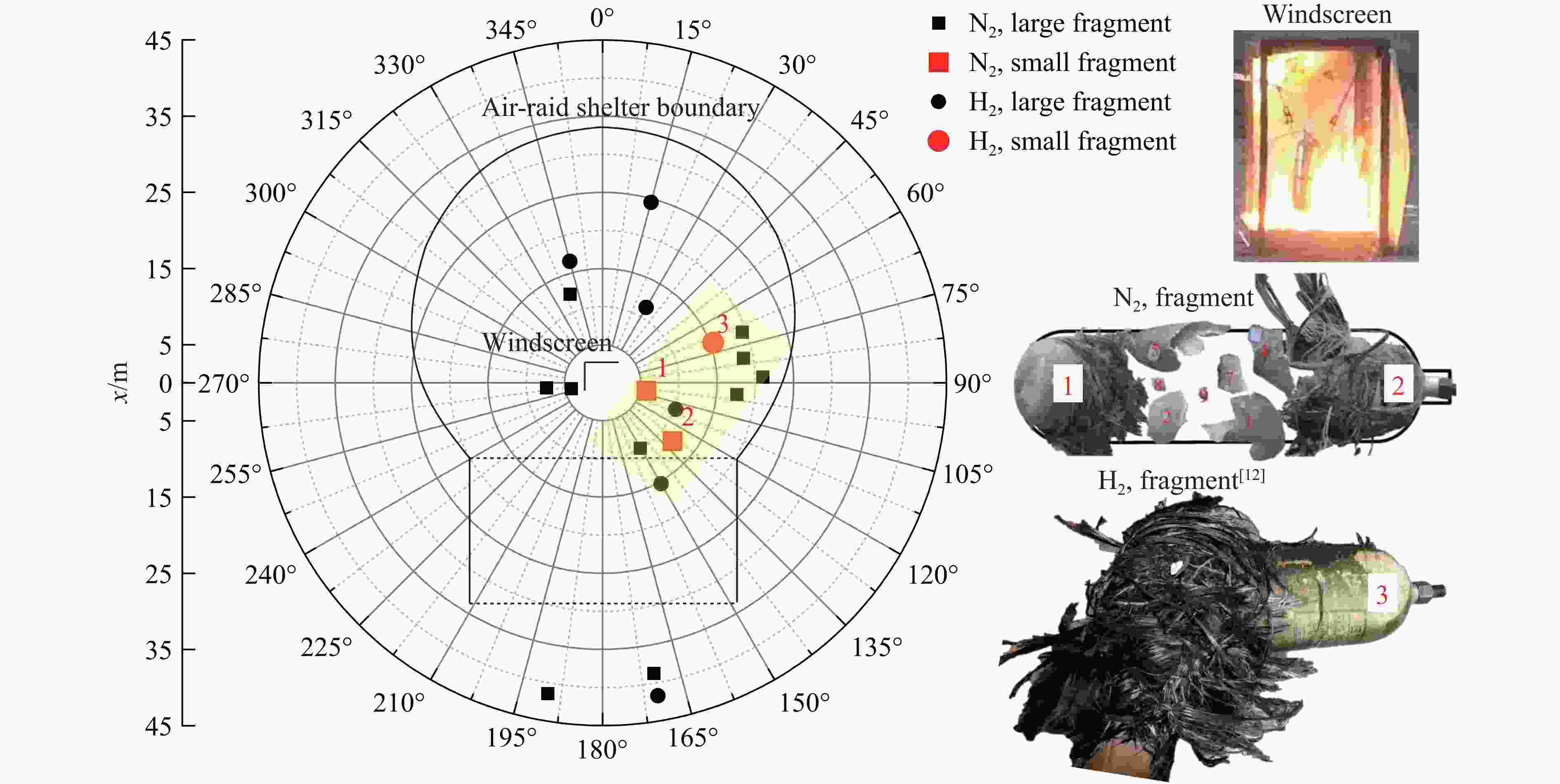
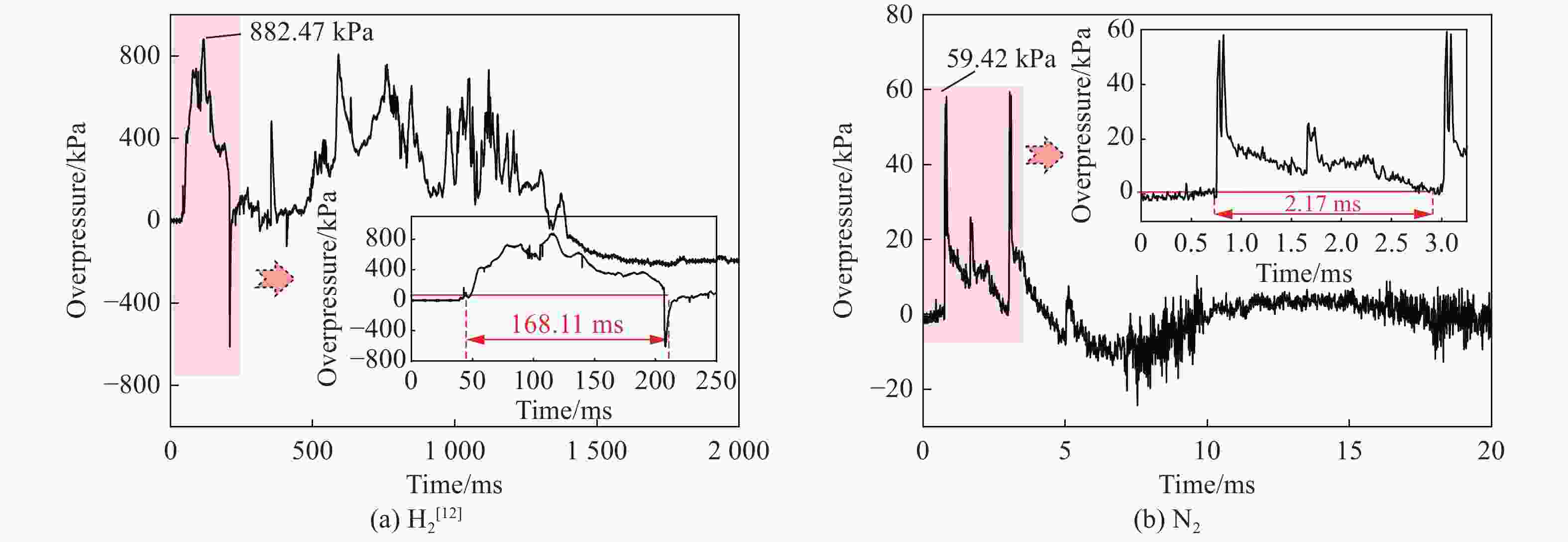
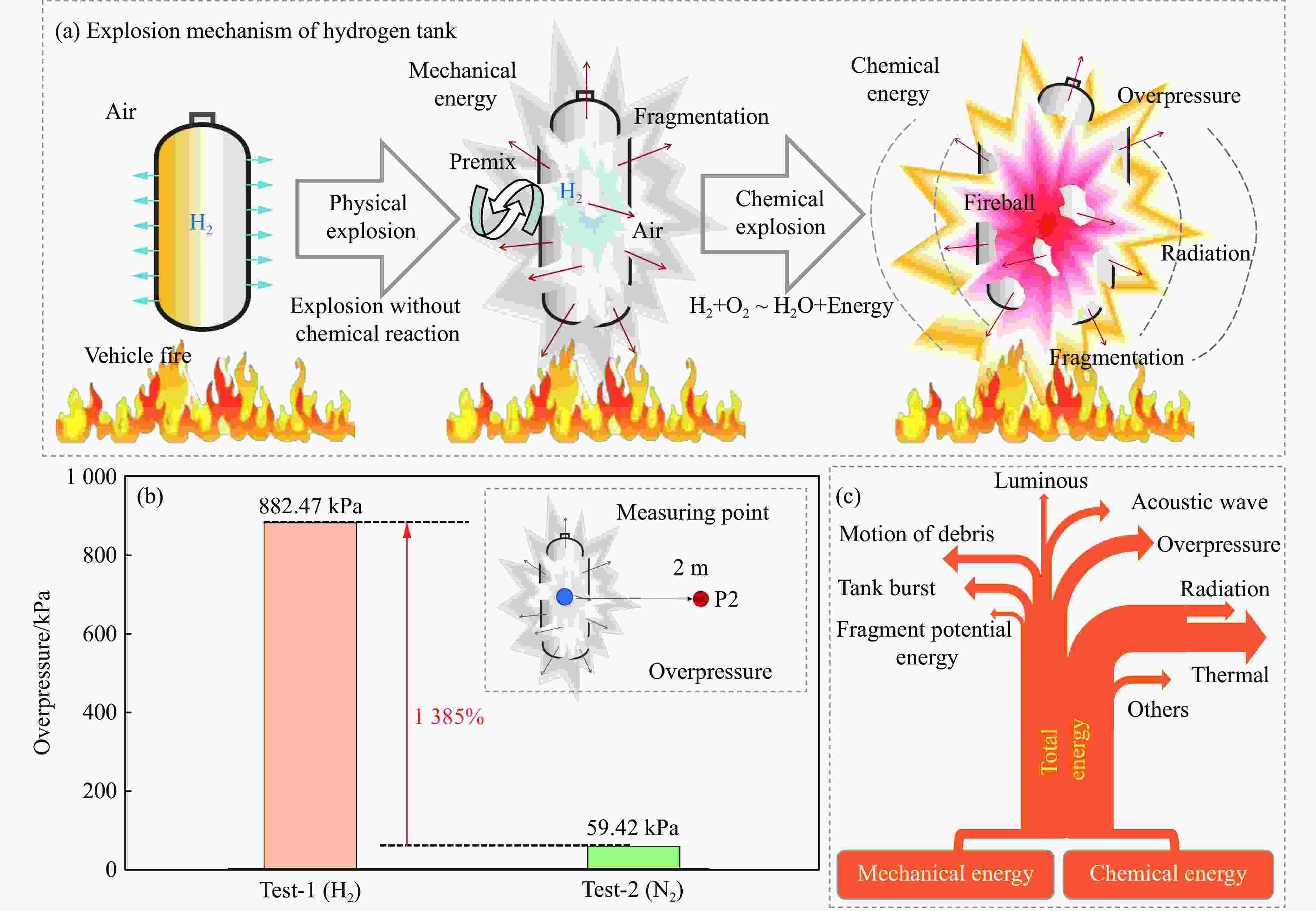
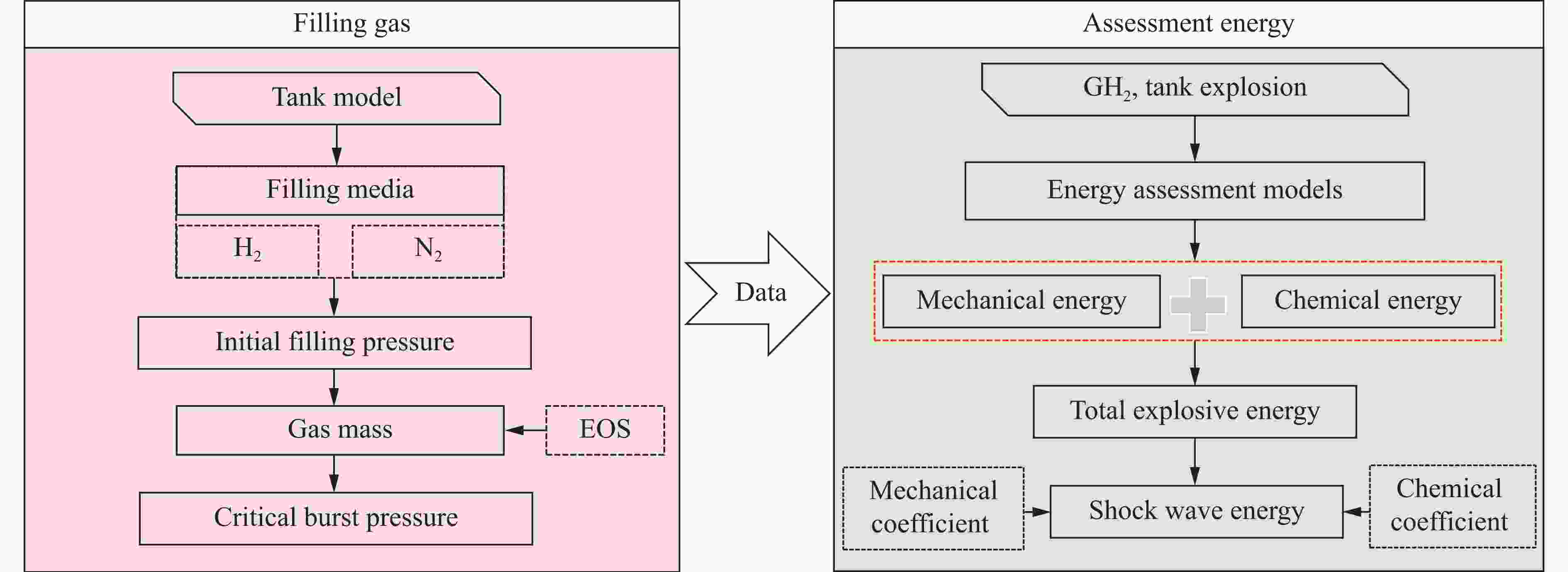
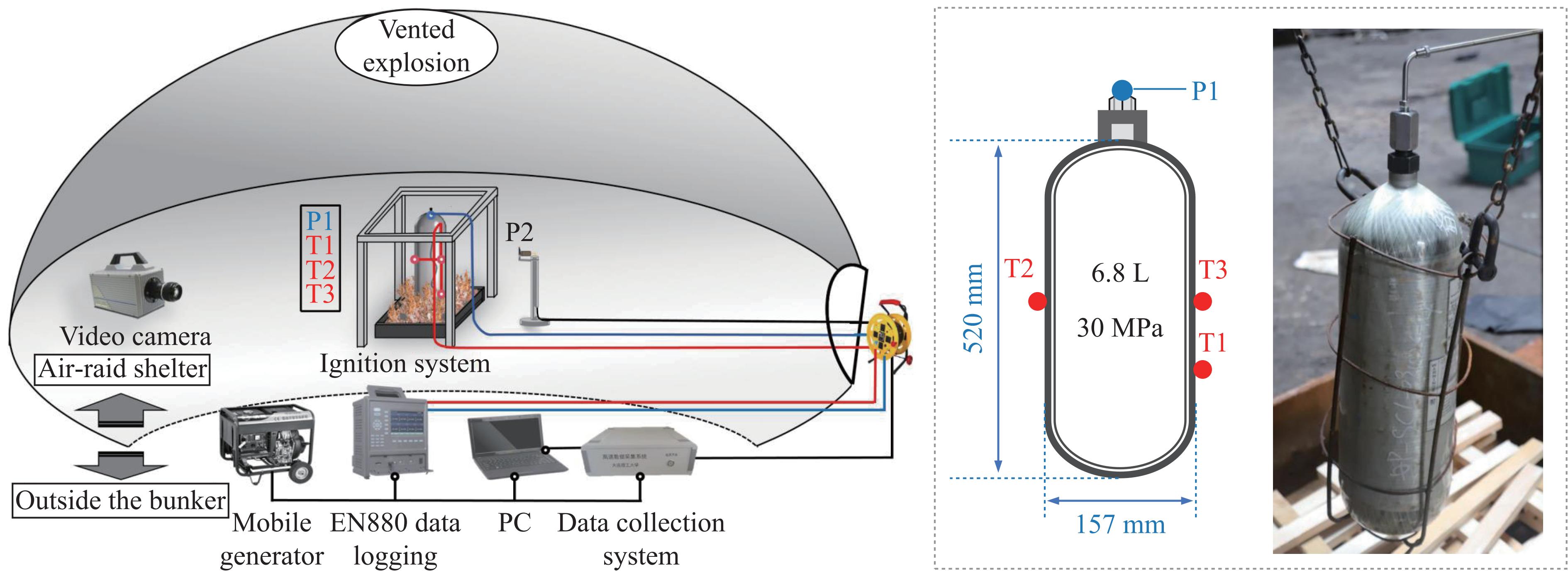
摘要: 为掌握火灾环境下高压储氢气瓶爆炸能量产生、转化及耗散机制对气瓶爆炸的影响,以充装氢气和氮气的6.8L-30MPa Ⅲ型高压气瓶爆炸引发试验为基础,开展了气瓶极限承压判据、爆炸动力学行为及威力评估研究。结果表明:火灾可显著降低气瓶承压能力,气瓶的临界爆破压力由常温时的125.1 MPa降至火灾时的46.8 MPa,承压能力下降约62.6%。储氢气瓶爆炸呈现典型的物理-化学复合特征,产生了直径约9 m的火球,冲击波峰值压力在距离爆源2 m处达882.47 kPa,正压持续时间为168.11 ms;相同位置处的氮气瓶爆炸冲击波峰值为59.42 kPa,正压持续时间为2.17 ms,爆炸威力远小于氢气瓶。探讨了开敞环境下氢气瓶与氮气瓶爆炸能量的转化路径,建立了开敞环境下储氢气瓶爆炸威力评估方法,研究结果可为完善高压储氢气瓶爆炸事故风险评估提供参考。Abstract: Understanding the generation, transformation, and dissipation mechanisms of energy in high-pressure tanks during fire scenarios is of critical significance for the consequence assessment of explosion accidents. This study investigates the differences in properties between high-pressure hydrogen storage tanks and nitrogen tanks under fire conditions through comparative experiments. Fire tests were conducted using 6.8L-30MPa type Ⅲ tanks. The results indicate that fire exposure can significantly impair the pressure-bearing capacity of the tanks. Specifically, the critical bursting pressure decreased from 125.1 MPa at room temperature to 46.8 MPa under fire conditions, representing a reduction of 62.6%. The explosion dynamics of hydrogen tanks were characterized by typical physical-chemical composite features. A fireball with a diameter of 9 m was formed during the explosion. The peak shockwave pressure measured at a distance of 2 m reached 882.47 kPa, with a positive pressure duration of 168.11 ms. In contrast, nitrogen tanks experienced only physical explosions, with a peak shockwave pressure of 59.42 kPa and a positive pressure duration of merely 2.17 ms. This study analyzed the energy conversion pathways during explosions of high-compressed gas tanks (H2 and N2) in open environments. A novel method for assessing the blast power of hydrogen storage cylinder explosions in unconfined spaces was developed. Initially, the physical explosion energy was calculated based on fundamental parameters such as critical burst pressure, nominal volume, and initial filling pressure of the high-pressure tanks. The applicability of five mechanical energy calculation models was compared. Subsequently, the mass of hydrogen was determined using the actual gas equation, and the total chemical explosion energy was derived by integrating the heat of combustion of hydrogen. Finally, considering the contributions of mechanical and chemical energy to the shock wave intensity, the total explosion energy was converted into shock wave energy using an open space energy correction factor. Quantitative analysis and error verification were conducted in conjunction with measured data. The findings of this research provide essential support for enhancing risk assessment of explosion accidents involving high-pressure hydrogen storage devices.
-
Key words:
- explosion /
- hydrogen storage tank /
- type Ⅲ tank /
- hazards assessment
-
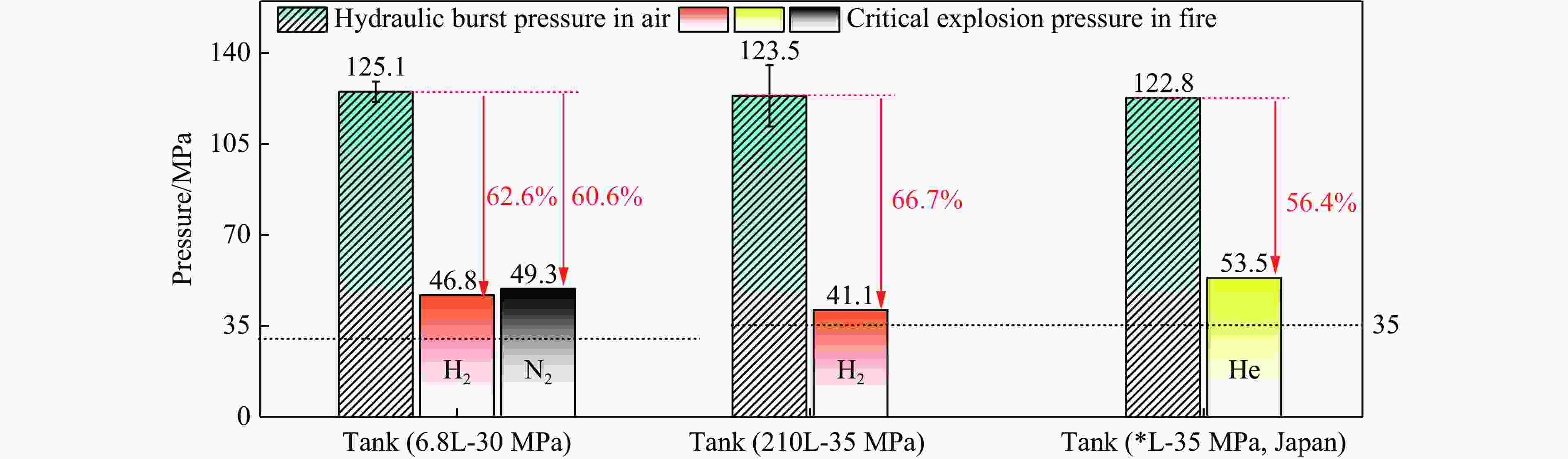
表 1 火灾环境下典型Ⅲ型瓶极限承压判据
Table 1. Criteria for the pressure-bearing limit of typical type Ⅲ tanks
公称容积-公称工作压力 6.8L-30MPa 6.8L-30MPa 210L-35MPa *L-35MPa 数据来源 文献[12] 本研究 文献[2] 文献[8] 气瓶水爆压力/MPa 125.07(±3.85) 125.07(±3.85) 123.5(±11.75) 122.8 火源 油池 油池 油池 丙烷燃烧器 火烧燃爆引发方式 整体 整体 局部+整体 整体 放置方式 垂直 垂直 水平 水平 充装介质 H2 N2 H2 He 初始充装压力/MPa 30.58 29.98 32.20 25.00 爆炸临界压力/MPa 46.8 49.3 41.1 53.5 火灾中承压性能下降幅度/% 62.6 60.6 66.7 56.4 注:文献[8]为日本汽车研究所气瓶火烧爆炸试验研究数据,气瓶编号为9#,未查阅到公称容积等信息。 表 2 气瓶爆炸超压能量计算结果
Table 2. Tank explosion overpressure energy calculation results
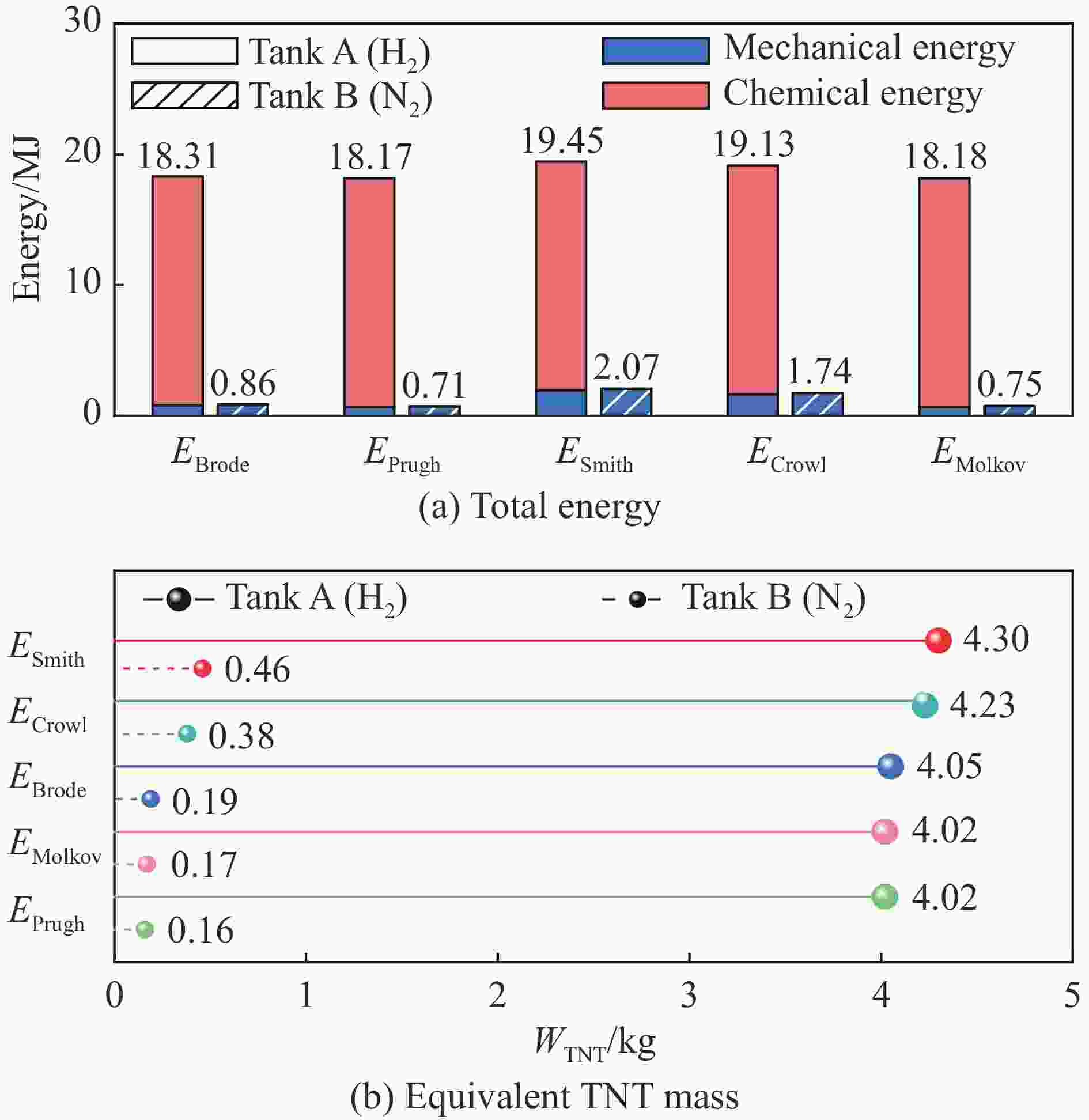
气瓶 介质 pb/MPa $m_{{\mathrm{H}}_2} $/kg Eam/MJ Eac/MJ Etotal/MJ WTNT/kg 气瓶A
(6.8L-30MPa)氢气 46.8 0.15 1.46 (EBrode) 0.91 2.37 0.52 1.21 (EPrugh) 2.12 0.47 3.51 (ESmith) 4.42 0.98 2.94 (ECrowl) 3.85 0.85 1.22 (EMolkov) 2.13 0.47 气瓶B
(6.8L-30MPa)氮气 49.3 0.11 1.55 (EBrode) 0 1.55 0.34 1.28 (EPrugh) 1.28 0.28 3.73 (ESmith) 3.73 0.83 3.13 (ECrowl) 3.13 0.69 1.46 (EMolkov) 1.46 0.32 气瓶C[19]
(165L-35MPa)氢气 44.0 3.89 33.43 (EBrode) 23.74 57.17 12.65 27.41 (EPrugh) 51.15 11.32 79.37 (ESmith) 103.11 22.81 66.33 (ECrowl) 90.07 19.93 27.37 (EMolkov) 51.11 11.31 21.32 (文献[19]) 24.04 45.36 10.04 -
[1] GORDON J A, BALTA-OZKAN N, NABAVI S A. Beyond the triangle of renewable energy acceptance: the five dimensions of domestic hydrogen acceptance [J]. Applied Energy, 2022, 324: 119715. DOI: 10.1016/j.apenergy.2022.119715. [2] LI B, HAN B, LI Q, et al. Study on hazards from high-pressure on-board type Ⅲ hydrogen tank in fire scenario: consequences and response behaviours [J]. International Journal of Hydrogen Energy, 2022, 47(4): 2759–2770. DOI: 10.1016/j.ijhydene.2021.10.205. [3] 郑津洋, 胡军, 韩武林, 等. 中国氢能承压设备风险分析和对策的几点思考 [J]. 压力容器, 2020, 37(6): 39–47. DOI: 10.3969/i.issn.1001-4837.2020.06.007.ZHENG J Y, HU J, HAN W L, et al. Risk analysis and some countermeasures of pressure equipment for hydrogen energy in China [J]. Pressure Vessel Technology, 2020, 37(6): 39–47. DOI: 10.3969/i.issn.1001-4837.2020.06.007. [4] 邹林志, 李贝, 韩冰, 等. 氢燃料电池车紧急泄放射流火辐射风险分析 [J]. 清华大学学报(自然科学版), 2025: 1–8. DOI: 10.16511/j.cnki.qhdxxb.2025.27.001.ZOU L Z, LI B, HAN B, et al. Risk prediction of thermal radiation from jet fire of on board hydrogen storage tanks [J]. Journal of Tsinghua University (Science and Technology), 2025: 1–8. DOI: 10.16511/j.cnki.qhdxxb.2025.27.001. [5] 曹湘洪, 魏志强. 氢能利用安全技术研究与标准体系建设思考 [J]. 中国工程科学, 2020, 22(5): 144–151. DOI: 10.15302/J-SSCAE-2020.05.018.CAO X H, WEI Z Q. Technologies for the safe use of hydrogen and construction of the safety standards system [J]. Strategic Study of CAE, 2020, 22(5): 144–151. DOI: 10.15302/J-SSCAE-2020.05.018. [6] ZALOSH R. Blast waves and fireballs generated by hydrogen fuel tank rupture during fire exposure [C]//Proceedings of the 5th International Seminar on Fire and Explosion Hazards. Wellesley College, US, 2007: 23–27. [7] HUPP N, STAHL U, KUNZE K, et al. Influence of fire intensity, fire impingement area and internal pressure on the fire resistance of composite pressure vessels for the storage of hydrogen in automobile applications [J]. Fire Safety Journal, 2019, 104: 1–7. DOI: 10.1016/j.firesaf.2018.12.004. [8] TAMURA Y, YAMAZAKI K, MAEDA K, et al. The residual strength of automotive hydrogen cylinders after exposure to flames [J]. International Journal of Hydrogen Energy, 2019, 44(17): 8759–8766. DOI: 10.1016/j.ijhydene.2018.12.055. [9] KUDRIAKOV S, STUDER E, BERNARD-MICHEL G, et al. Full-scale tunnel experiments: blast wave and fireball evolution following hydrogen tank rupture [J]. International Journal of Hydrogen Energy, 2022, 47(43): 18911–18933. DOI: 10.1016/j.ijhydene.2022.04.037. [10] MONTIEL H, VÍLCHEZ J A, CASAL J, et al. Mathematical modelling of accidental gas releases [J]. Journal of Hazardous Materials, 1998, 59(2/3): 211–233. DOI: 10.1016/S0304-3894(97)00149-0. [11] 李桐, 陈明, 叶志伟, 等. 不同耦合介质爆破爆炸能量传递效率研究 [J]. 爆炸与冲击, 2021, 41(6): 062201. DOI: 10.11883/bzycj-2020-0381.LI T, CHEN M, YE Z W, et al. Study on the energy transfer efficiency of explosive blasting with different coupling medium [J]. Explosion and Shock Waves, 2021, 41(6): 062201. DOI: 10.11883/bzycj-2020-0381. [12] WANG X Y, LI B, HAN B, et al. Explosion of high pressure hydrogen tank in fire: mechanism, criterion, and consequence assessment [J]. Journal of Energy Storage, 2023, 72: 108455. DOI: 10.1016/j.est.2023.108455. [13] MOLKOV V V, CIRRONE D M C, SHENTSOV V V, et al. Dynamics of blast wave and fireball after hydrogen tank rupture in a fire in the open atmosphere [J]. International Journal of Hydrogen Energy, 2021, 46(5): 4644–4665. DOI: 10.1016/j.ijhydene.2020.10.211. [14] CIRRONE D, MAKAROV D, MOLKOV V. Rethinking “BLEVE explosion” after liquid hydrogen storage tank rupture in a fire [J]. International Journal of Hydrogen Energy, 2023, 48(23): 8716–8730. DOI: 10.1016/j.ijhydene.2022.09.114. [15] 瞿飞. 氢燃料电池汽车灭火救援的对策研究 [J]. 中国消防, 2024(5): 60–63. DOI: 10.3969/j.issn.1672-9668.2019.02.042.QU F. Research on countermeasures for fire fighting and rescue of hydrogen fuel cell vehicle [J]. China Fire, 2024(5): 60–63. DOI: 10.3969/j.issn.1672-9668.2019.02.042. [16] 李贝, 王雪莹, 金鑫, 等. 火灾场景下碳纤维复合高压储氢装置热损伤特征研究 [J]. 太阳能学报, 2022, 43(6): 398–404. DOI: 10.19912/j.0254-0096.tynxb.2022-0421.LI B, WANG X Y, JIN X, et al. Study on thermal damage properties of carbon fiber composite high-pressure hydrogen tank under fire scenarios [J]. Acta Energiae Solaris Sinica, 2022, 43(6): 398–404. DOI: 10.19912/j.0254-0096.tynxb.2022-0421. [17] MOLKOV V, KASHKAROV S. Blast wave from a high-pressure gas tank rupture in a fire: stand-alone and under-vehicle hydrogen tanks [J]. International Journal of Hydrogen Energy, 2015, 40(36): 12581–12603. DOI: 10.1016/j.ijhydene.2015.07.001. [18] ZHAO X L, CHEN C K, SHI C L, et al. A three-dimensional simulation of the effects of obstacle blockage ratio on the explosion wave in a tunnel [J]. Journal of Thermal Analysis and Calorimetry, 2021, 143(4): 3245–3256. DOI: 10.1007/s10973-020-09777-7. [19] SHEN C C, MA L, HUANG G, et al. Consequence assessment of high-pressure hydrogen storage tank rupture during fire test [J]. Journal of Loss Prevention in the Process Industries, 2018, 55: 223–231. DOI: 10.1016/j.jlp.2018.06.016. [20] BAKER W E, COX P A, WESTINE P S, et al. Explosion hazards and evaluation [M]. Amsterdam: Elsevier, 1983. [21] 曹涛, 孙浩, 周游, 等. 近地爆炸冲击波传播特性数值模拟与应用 [J]. 兵器装备工程学报, 2020, 41(12): 187–191. DOI: 10.11809/bqzbgcxb2020.12.035.CAO T, SUN H, ZHOU Y, et al. Numerical simulation and application of propagation characteristics of shock wave near ground explosion [J]. Journal of Ordnance Equipment Engineering, 2020, 41(12): 187–191. DOI: 10.11809/bqzbgcxb2020.12.035. [22] KASHKAROV S, LI Z Y, MOLKOV V. Blast wave from a hydrogen tank rupture in a fire in the open: hazard distance nomograms [J]. International Journal of Hydrogen Energy, 2020, 45(3): 2429–2446. DOI: 10.1016/j.ijhydene.2019.11.084. -






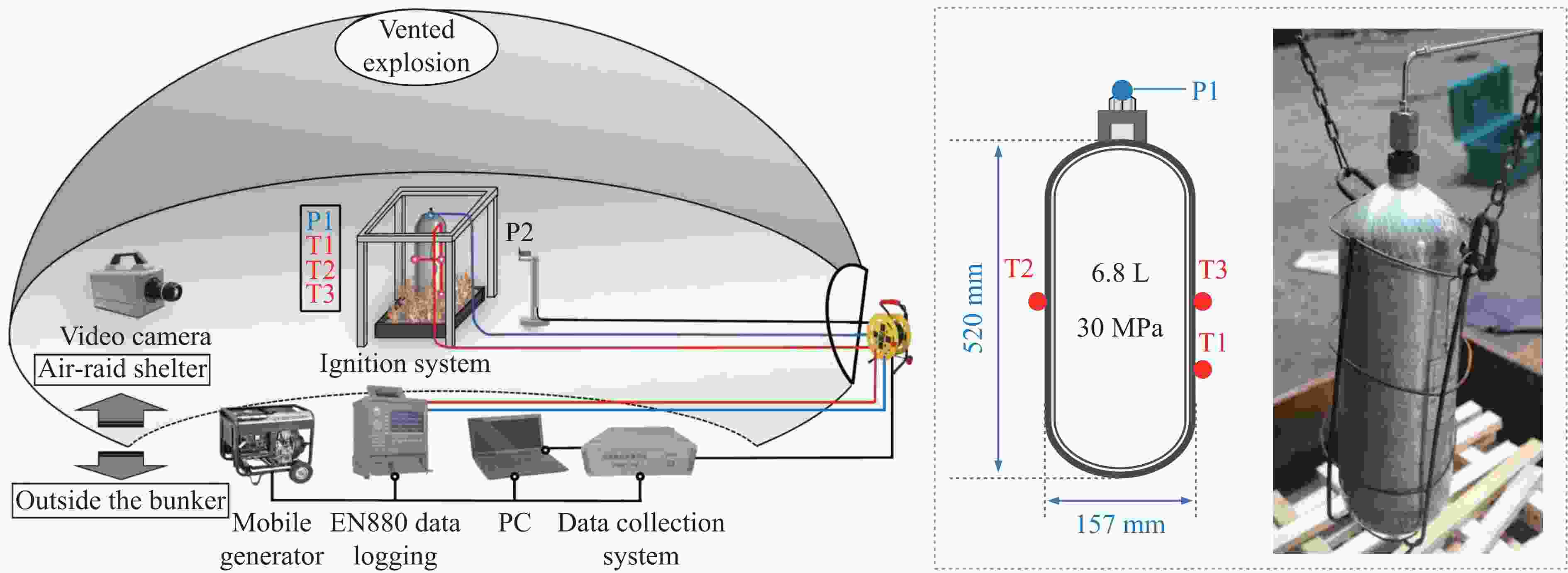
 下载:
下载: